Clin Exp Otorhinolaryngol.
2017 Jun;10(2):181-187. 10.21053/ceo.2016.00262.
Effect of Hippophae rhamnoides Extract on Oxidative Oropharyngeal Mucosal Damage Induced in Rats Using Methotrexate
- Affiliations
-
- 1Department of Otorhinolaryngology, Faculty of Medicine, Erzincan University, Erzincan, Turkey. dreerhan@hotmail.com
- 2Department of Otorhinolaryngology, Faculty of Medicine, Recep Tayyip Erdogan University, Rize, Turkey.
- 3Department of Otorhinolaryngology, Rize Education and Research Hospital, Rize, Turkey.
- 4Department of Medical Genetics, Erzurum Training and Research Hospital, Erzurum, Turkey.
- 5Department of Biology, Faculty of Arts and Sciences, Erzincan University, Erzincan, Turkey.
- 6Department of Pathology, Mengucek Gazi Education and Research Hospital, Erzincan, Turkey.
- 7Department of Anatomy, Faculty of Medicine, Ataturk University, Erzurum, Turkey.
- 8Department of Pharmacology, Faculty of Medicine, Erzincan University, Erzincan, Turkey.
- KMID: 2380438
- DOI: http://doi.org/10.21053/ceo.2016.00262
Abstract
OBJECTIVES
The objective of this study is to investigate and evaluate the effect of Hippophae rhamnoides extract (HRE) on oropharyngeal mucositis induced in rats with methotrexate (MTX) through biochemical, gene expression, and histopathological examinations.
METHODS
Experimental animals were divided into a healthy group (HG), a HRE+MTX (HREM) group, HRE group (HREG), and a control group that received MTX (MTXG). The HREM and HREG groups of rats was administered 50 mg/kg HRE, while the MTXG and HG groups were given an equal volume distilled water with gavage. Then, the HREM and MTXG rat groups were given oral MTX at a dose of 5 mg/kg 1 hour after HRE and distilled water was administered. This procedure was repeated for 1 month. At the end of this period, all of the animals were sacrificed with a high dose of anesthesia. Then, the amounts of malondialdehyde (MDA) and total glutathione (tGSH) were determined in the removed oropharyngeal tissues. Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) gene expressions were measured, and all the tissues were studied histopathologically.
RESULTS
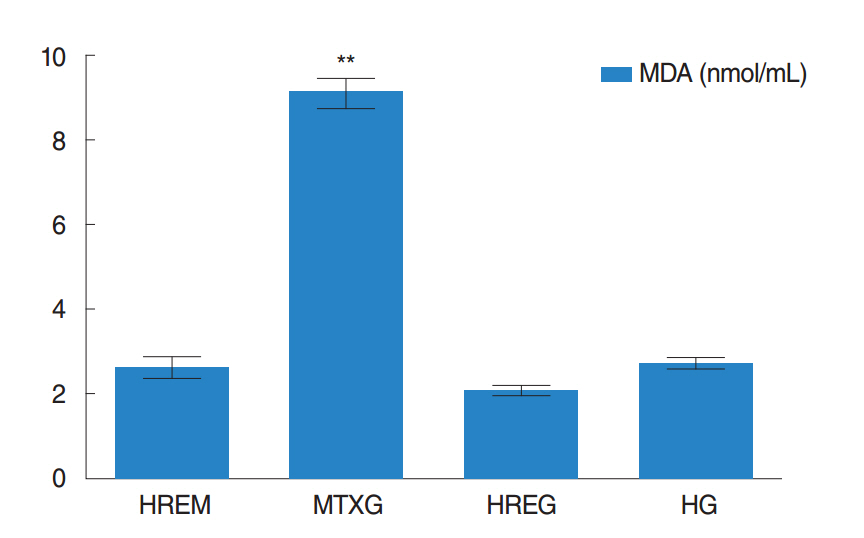
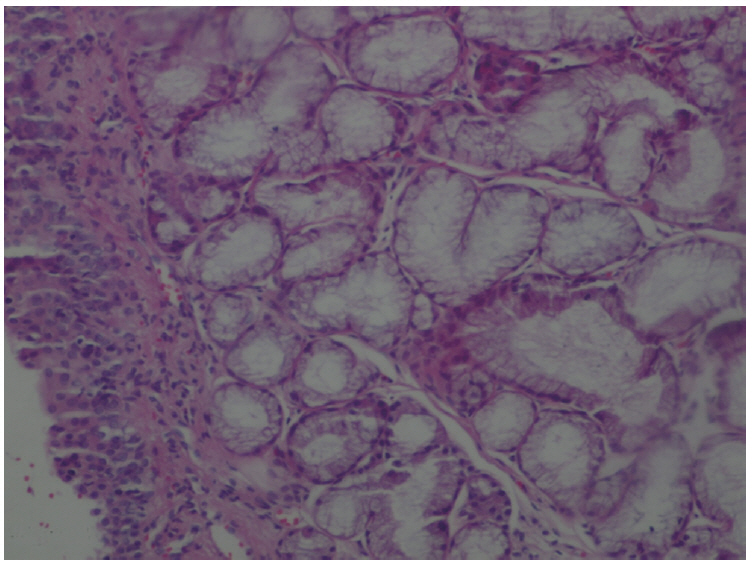
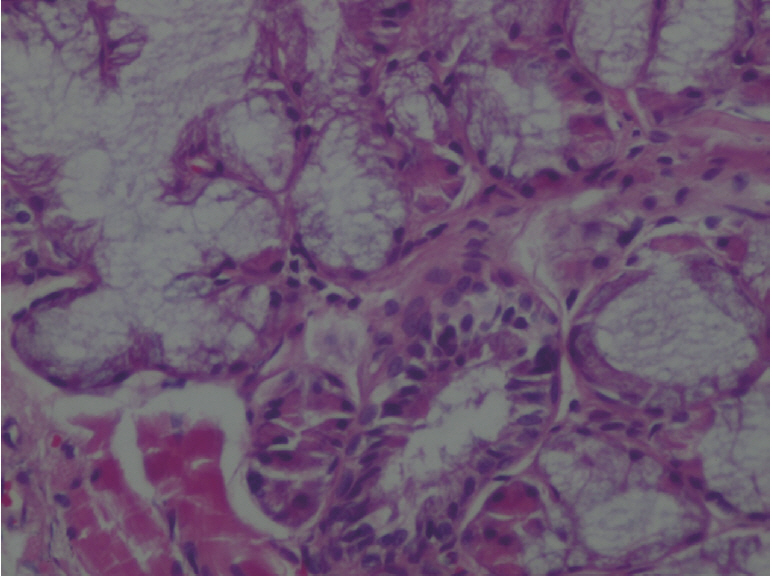
The amount of MDA was significantly increased in the MTXG group compared to the HREM, HREG, and HG groups (P<0.001). MTX significantly decreased the amount of tGSH in the MTXG group compared to the HREM, HREG, and HG groups (P<0.001). In this study, there were no visible ulcers in the animal group in which the levels of MDA, IL-1β, and TNF-α were high and the level of tGSH was low. However, histopathologic examination revealed mucin pools in wide areas due to ruptured oropharynx glands, and proliferated, dilated, and congested blood vessels and dilated ductal structures in some areas.
CONCLUSION
HRE protected oropharyngeal oxidative damage induced by MTX. As an inexpensive and natural product, HRE has important advantages in the prevention of oropharyngeal damage induced by MTX.
Keyword
MeSH Terms
Figure
Reference
-
1. Kwong KK. Prevention and treatment of oropharyngeal mucositis following cancer therapy: are there new approaches. Cancer Nurs. 2004; May-Jun. 27(3):183–205.2. Weisdorf DJ, Bostrom B, Raether D, Mattingly M, Walker P, Pihlstrom B, et al. Oropharyngeal mucositis complicating bone marrow transplantation: prognostic factors and the effect of chlorhexidine mouth rinse. Bone Marrow Transplant. 1989; Jan. 4(1):89–95.3. Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009; Dec. 45(12):1015–20.
Article4. Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck. 2003; Dec. 25(12):1057–70.
Article5. Ahmed KM. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer: a randomized clinical trial. Saudi Dent J. 2013; Oct. 25(4):141–7.
Article6. Mardani M, Afra SM, Tanideh N, Tadbir AA, Modarresi F, Koohi-Hosseinabadi O, et al. Hydroalcoholic extract of Carum carvi L. in oral mucositis: a clinical trial in male golden hamsters. Oral Dis. 2016; Jan. 22(1):39–45.7. de Araujo RF Jr, Reinaldo MP, Brito GA, Cavalcanti Pde F, Freire MA, de Medeiros CA, et al. Olmesartan decreased levels of IL-1β and TNF-α, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One. 2014; Dec. 9(12):e114923.
Article8. Vokurka S. Oropharyngeal mucositis: pain management. Klin Onkol. 2011; 24(4):278–80.9. Epstein JB, Tsang AH, Warkentin D, Ship JA. The role of salivary function in modulating chemotherapy-induced oropharyngeal mucositis: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; Jul. 94(1):39–44.
Article10. Brown CG, Yoder LH. Stomatitis: an overview: protecting the oral cavity during cancer treatment. Am J Nurs. 2002; Apr. 102 Suppl 4:20–3.11. Altindag O, Kucukoglu B. Intoxication due to high dose methotrexate in a patient with rheumatoid arthritis: a case report. Turk J Rheumatol. 2011; 26(1):58–60.
Article12. Vanhoecke B, Bateman E, Mayo B, Vanlancker E, Stringer A, Thorpe D, et al. Dark Agouti rat model of chemotherapy-induced mucositis: establishment and current state of the art. Exp Biol Med (Maywood). 2015; Jun. 240(6):725–41.
Article13. Ilgenli T, Oren H, Uysal K. The acute effects of chemotherapy upon the oral cavity: prevention and management. Turk J Cancer. 2001; Jul. 31(3):93–105.14. Jahovic N, Sener G, Cevik H, Ersoy Y, Arbak S, Yegen BC. Amelioration of methotrexate-induced enteritis by melatonin in rats. Cell Biochem Funct. 2004; May-Jun. 22(3):169–78.
Article15. Alamir I, Boukhettala N, Aziz M, Breuille D, Dechelotte P, Coeffier M. Beneficial effects of cathepsin inhibition to prevent chemotherapy-induced intestinal mucositis. Clin Exp Immunol. 2010; Nov. 162(2):298–305.
Article16. Suleyman H, Demirezer LO, Buyukokuroglu ME, Akcay MF, Gepdiremen A, Banoglu ZN, et al. Antiulcerogenic effect of Hippophae rhamnoides L. Phytother Res. 2001; Nov. 15(7):625–7.17. Andersson SC, Rumpunen K, Johansson E, Olsson ME. Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J Agric Food Chem. 2008; Aug. 56(15):6701–6.
Article18. Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY, Park J. Casuarinin suppresses TNF-α-induced ICAM-1 expression via blockade of NF-κB activation in HaCaT cells. Biochem Biophys Res Commun. 2011; Jun. 409(4):780–5.
Article19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; Jun. 95(2):351–8.
Article20. Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968; Oct. 24. 25(1):192–205.
Article21. Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 2013; Feb. 45(1):47–9.
Article22. Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000; Aug. 72(2 Suppl):637S–46S.
Article23. Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. 2004; Oct. 430(1):97–103.
Article24. Aguilar A, Alvarez-Vijande R, Capdevila S, Alcoberro J, Alcaraz A. Antioxidant patterns (superoxide dismutase, glutathione reductase, and glutathione peroxidase) in kidneys from non-heart-beating-donors: experimental study. Transplant Proc. 2007; Jan-Feb. 39(1):249–52.
Article25. Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006; Aug. 12(8):BR274–8.26. Arslan A, Ozcicek F, Keskin Cimen F, Altuner D, Yarali O, Kurt N, et al. Protective effect of resveratrol against methotrexate-induced oxidative stress in the small intestinal tissues of rats. Int J Clin Exp Med. 2015; Jul. 8(7):10491–500.27. Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered. Cancer Chemother Pharmacol. 2009; Jan. 63(2):239–51.
Article28. de Araujo AA, Borba PB, de Souza FH, Nogueira AC, Saldanha TS, Araujo TE, et al. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol Pharm Bull. 2015; 38(5):746–52.29. Mills EE. The modifying effect of beta-carotene on radiation and chemotherapy induced oral mucositis. Br J Cancer. 1988; Apr. 57(4):416–7.
Article30. Yilmaz I, Demiryilmaz I, Sener E, Cetin N, Ucuncu Y, Altuner D, et al. The effect of hippophae rhamnoides extract on oxidative damage on rat’s gastric tissue depending on co-implementation of methotrexate and indomethacin. Lat Am J Pharm. 2014; 33(3):453–8.31. Suryakumar G, Gupta A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol. 2011; Nov. 138(2):268–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analgesic Effect of Hippophae rhamnoides Extract in Orofacial Pain in Rats
- The Role of Neutrophil on Nonsteroidal Anti-Inflammatory Drugs Induced Acute Gastric Mucosal Injury
- Protective effect of an ethanol extract mixture of Aralia elata, Chaenomeles sinensis fruit, and Glycyrrhizae radix against cerebral ischemiareperfusion injury in rats and excitotoxic and oxidative neuronal damage
- Effect of lutein on methotrexate-induced oxidative lung damage in rats: a biochemical and histopathological assessment
- The Improvement of Chaga Mushroom (Inonotus Obliquus) Extract Supplementation on the Blood Glucose and Cellular DNA Damage in Streptozotocin-Induced Diabetic Rats