Cancer Res Treat.
2015 Jan;47(1):72-77. 10.4143/crt.2013.172.
Changes in the Mean Corpuscular Volume after Capecitabine Treatment Are Associated with Clinical Response and Survival in Patients with Advanced Gastric Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hematoma@skku.edu
- 2Department of Obstetrics and Gynecology, Konkuk University Hospital, Konkuk University School of Medicine, Chungju, Korea.
- 3Division of Hematology-Oncology, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 2380392
- DOI: http://doi.org/10.4143/crt.2013.172
Abstract
- PURPOSE
Capecitabine is known to increase mean corpuscular volume (MCV). To define the incidence of capecitabine-induced macrocytosis and its association with chemotherapy outcomes, we investigated data of 89 patients with advanced gastric cancer (AGC) who were enrolled in a randomized chemotherapy trial involving capecitabine.
MATERIALS AND METHODS
Chemotherapy-naive AGC patients were treated with capecitabine (1,000 mg/m2/day on days 1-14) plus cisplatin (75 mg/m2 on day 1), with or without epirubicin (50 mg/m2 on day 1). Complete blood counts including MCV were measured at baseline and on day 1 of each 3-week chemotherapy course. Macrocytosis was defined as a MCV increase > 10 fL from baseline. Multivariate Cox proportional hazards models were used for analysis of the impact of clinical and MCV values on chemotherapy outcomes.
RESULTS
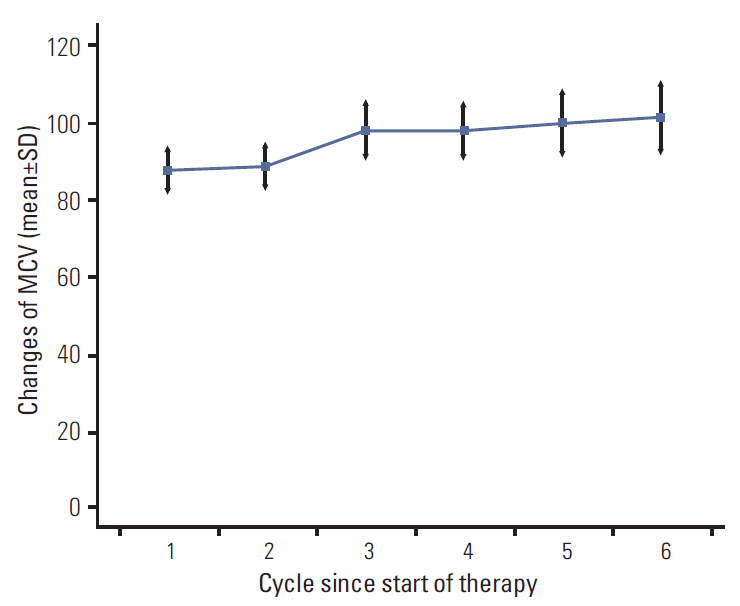
At baseline, the mean MCV was 88.2 fL (normal range, 80 to 100 fL). During chemotherapy, MCV increased in a dose-dependent manner with a mean increase of 11.3 fL. MCV elevation after capecitabine treatment in 74 patients (90%) and 44 patients (42%) developed macrocytosis.
RESULTS
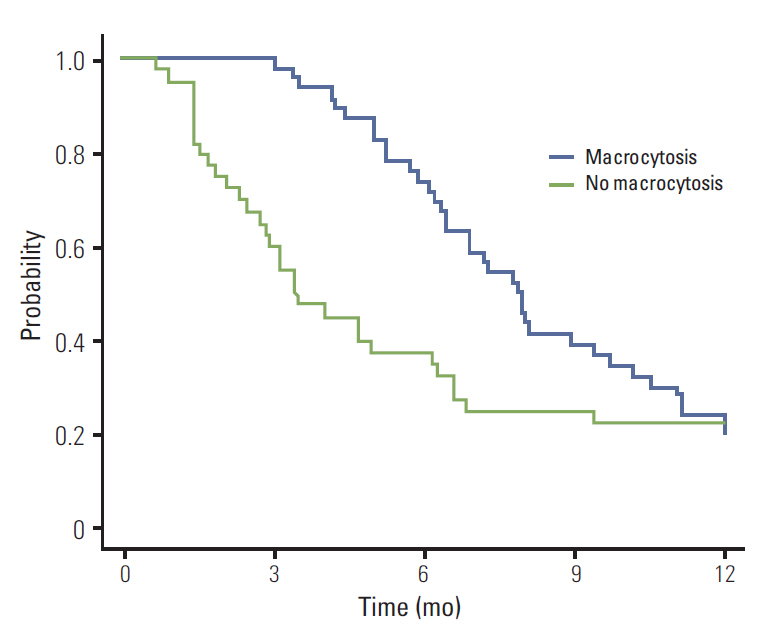
of multivariate analysis showed that development of macrocytosis was independent of baseline hemoglobin level, liver metastasis, performance status, or liver function. The number of chemotherapy cycles showed strong association with development of macrocytosis and hematologic adverse events. In addition, a significant association was observed between macrocytosis and clinical response or survival.
CONCLUSION
Macrocytosis developed with more frequent and prolonged use of capecitabine. It is possible that association with treatment outcomes warrants further investigation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Twelves C. Can capecitabine replace 5-FU/leucovorin in combination with oxaliplatin for the treatment of advanced colorectal cancer? Oncology (Williston Park). 2002; 16(12 Suppl No. 14):23–6.2. Okines A, Chau I, Cunningham D. Capecitabine in gastric cancer. Drugs Today (Barc). 2008; 44:629–40.
Article3. Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002; 38:349–58.4. Midgley R, Kerr DJ. Capecitabine: have we got the dose right? Nat Clin Pract Oncol. 2009; 6:17–24.
Article5. Karvellas CJ, Sawyer M, Hamilton M, Mackey JR. Effect of capecitabine on mean corpuscular volume in patients with metastatic breast cancer. Am J Clin Oncol. 2004; 27:364–8.
Article6. Sun JF, Wu RR, Norris C, Noone AM, Amankwa-Sakyi M, Slack R, et al. Safety of chronic low-dose capecitabine as maintenance therapy in gastrointestinal cancers. Gastrointest Cancer Res. 2009; 3:134–40.7. Wenzel C, Mader RM, Steger GG, Pluschnig U, Kornek GV, Scheithauer W, et al. Capecitabine treatment results in increased mean corpuscular volume of red blood cells in patients with advanced solid malignancies. Anticancer Drugs. 2003; 14:119–23.
Article8. Scott JM, Weir DG. Drug-induced megaloblastic change. Clin Haematol. 1980; 9:587–606.
Article9. Vrecl R, Gerold M, Melzer S, Rainer W, Weitzer W. Effect of capecitabine on mean corpuscular volume of red blood cells. Am J Clin Oncol. 2005; 28:534.
Article10. van Triest B, Pinedo HM, Blaauwgeers JL, van Diest PJ, Schoenmakers PS, Voorn DA, et al. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res. 2000; 6:1063–72.11. Yun J, Lee J, Park SH, Park JO, Park YS, Lim HY, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J Cancer. 2010; 46:885–91.
Article12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.13. Park SH, Lee J, Lee SH, Park JO, Kim K, Kim WS, et al. Anemia is the strongest prognostic factor for outcomes of 5-fluorouracil-based first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2006; 57:91–6.
Article14. Allegra CJ, Parr AL, Wold LE, Mahoney MR, Sargent DJ, Johnston P, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002; 20:1735–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A New Option for Advanced Gastric Cancer: Docetaxel and Novel Oral Fluoropyrimidine Combination Chemotherapy
- Long-term oncologic outcomes of neoadjuvant concurrent chemoradiotherapy with capecitabine and radical surgery in locally advanced rectal cancer: 10-year experiences at a single institution
- Feasibility of Gastric Cancer Surgery at Low Volume Hospitals
- Additional 4-week capecitabine during the resting periods after 6-week neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: long-term oncologic outcomes
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer