J Breast Cancer.
2017 Mar;20(1):108-111. 10.4048/jbc.2017.20.1.108.
Bilateral Triple-Negative Invasive Breast Cancer with a BRCA2 Mutation, and Glioblastoma: A Case Report and Literature Review
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Joan C. Edwards School of Medicine at Marshall University/Edward Comprehensive Care Center, Huntington, USA. raufi@marshall.edu
- KMID: 2379406
- DOI: http://doi.org/10.4048/jbc.2017.20.1.108
Abstract
- Breast cancer is the second leading cause of death among women in North America. Glioblastoma is the most common primary malignant central nervous system tumor in adults. The majority of hereditary breast cancers are associated with deleterious mutations in the BRCA1 and BRCA2 genes. Although few case reports have described the incidence of glioblastoma in patients previously diagnosed with breast cancer, any association between BRCA2 mutations and glioblastoma has not been demonstrated to date. Herein, we report a woman who is a carrier of a familial BRCA2 mutation, and was previously diagnosed with triple-negative breast cancer (TNBC) and subsequently with a second primary TNBC and glioblastoma. Further investigation is required to define the possible relationship between these two aggressive malignances and the BRCA2 mutation, which might be critical for the proper management and treatment of this disease.
MeSH Terms
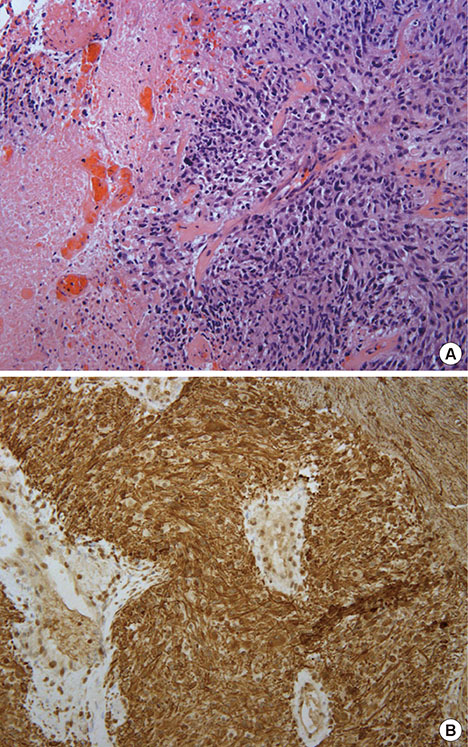
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Moran A, O'Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer. 2012; 11:235–242.
Article3. Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: the Breast Cancer Linkage Consortium. Am J Hum Genet. 1998; 62:676–689.
Article4. Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer. 2011; 11:325.
Article5. Varley JM, Evans DG, Birch JM. Li-Fraumeni syndrome: a molecular and clinical review. Br J Cancer. 1997; 76:1–14.
Article6. Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997; 275:1943–1947.
Article7. Gastaut JL, Koeppel MC, Alliez B, Michel B, Gambarelli D, Martin PM. Triple tumor association: breast cancer, meningioma and glioblastoma. Rev Neurol (Paris). 1987; 143:753–758.8. Piccirilli M, Salvati M, Bistazzoni S, Frati A, Brogna C, Giangaspero F, et al. Glioblastoma multiforme and breast cancer: report on 11 cases and clinico-pathological remarks. Tumori. 2005; 91:256–260.
Article9. Elmariah SB, Huse J, Mason B, Leroux P, Lustig RA. Multicentric glioblastoma multiforme in a patient with BRCA-1 invasive breast cancer. Breast J. 2006; 12:470–474.
Article10. Reid S, Renwick A, Seal S, Baskcomb L, Barfoot R, Jayatilake H, et al. Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet. 2005; 42:147–151.
Article11. Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet. 2007; 44:1–9.
Article12. Boukerroucha M, Josse C, Segers K, El-Guendi S, Frères P, Jerusalem G, et al. BRCA1 germline mutation and glioblastoma development: report of cases. BMC Cancer. 2015; 15:181.
Article13. Plunkett RJ, Lis A, Barone TA, Fronckowiak MD, Greenberg SJ. Hormonal effects on glioblastoma multiforme in the nude rat model. J Neurosurg. 1999; 90:1072–1077.
Article14. Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004; 64:6892–6899.15. McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ. The impact of age and sex on the incidence of glial tumors in New York State from 1976 to 1995. J Neurosurg. 2000; 93:932–939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Frequency of BRCA1 and BRCA2 Germline Mutations Detected by Protein Truncation Test and Cumulative Risks of Breast and Ovarian Cancer among Mutation Carriers in Japanese Breast Cancer Families
- Experience with Bilateral Risk-Reducing Mastectomy for an Unaffected BRCA Mutation Carrier
- Bilateral Triple Negative Invasive Ductal Breast Carcinoma in a BRCA1 Mutation Carrier with Discrepant Pathologic Response to Neoadjuvant Chemotherapy
- Novel Germline Mutations of BRCA1 and BRCA2 in Korean Familial Breast Cancer Patients
- Endometrial cancer occurence five years after breast cancer in BRCA2 mutation patient