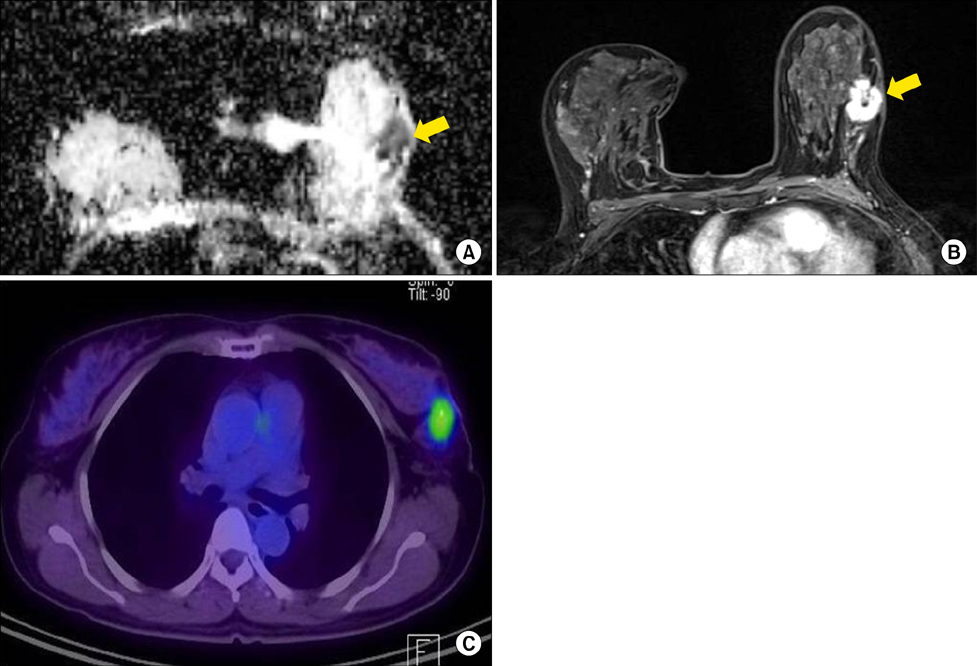
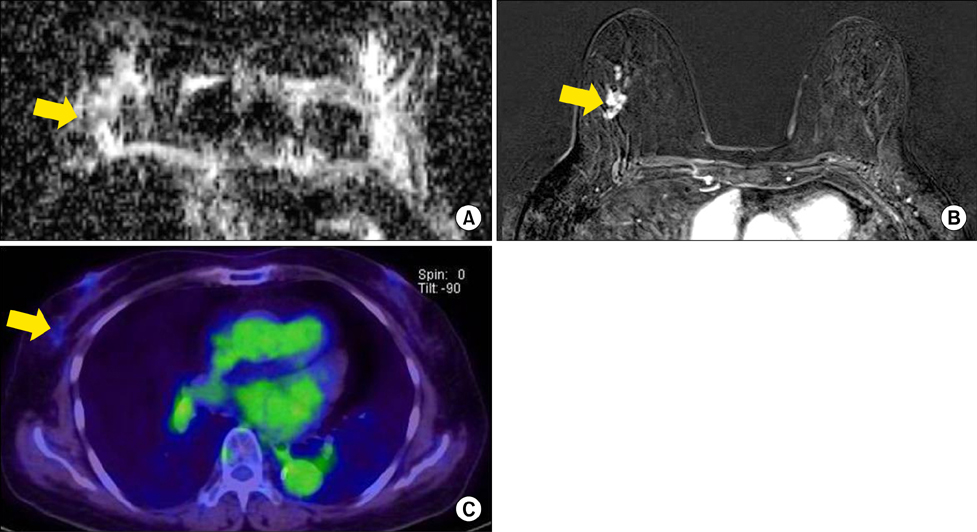
Chonnam Med J.
2017 May;53(2):133-139. 10.4068/cmj.2017.53.2.133.
Correlation of Prognostic Factors of Invasive Lobular Carcinoma with ADC Value of DWI and SUVMax of FDG-PET
- Affiliations
-
- 1Department of Radiology, Chungnam University Hospital, Daejeon, Korea.
- 2Department of Radiology, Seoul St. Mary' Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. rad-ksh@catholic.ac.kr
- 3Department of Radiology, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 4Department of Radiology, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea.
- KMID: 2379286
- DOI: http://doi.org/10.4068/cmj.2017.53.2.133
Abstract
- Invasive lobular carcinoma (ILC) is the second most common kind of breast cancer. Diffusion weighted imaging (DWI) and positron emission tomography/computed tomography (PET/CT) are functional modalities for presenting the biological characteristics of breast cancer. The purpose of this article is to study the relationship between DWI or PET/CT and ILC's prognostic factors. The relationship between the apparent diffusion coefficient (ADC) values, standard uptake value (SUV)max and prognostic factors of ILC were statistically evaluated. The ADC values were lower in mass types of ILC. SUVmax was statistically higher in grade 3 and 4 background parenchymal enhancement and positive lymph node metastasis. ADC values of DWI and SUVmax of PET/CT can be helpful in the prediction of the prognosis of ILC.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging. 2009; 30:615–620.
Article2. Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009; 36:200–208.
Article3. Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging. 2011; 33:102–109.
Article4. Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010; 37:2011–2020.
Article5. Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998; 82:2227–2234.
Article6. Ikenaga N, Otomo N, Toyofuku A, Ueda Y, Toyoda K, Hayashi T, et al. Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg. 2007; 73:1151–1157.
Article7. Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979; 3:467–488.
Article8. Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975; 36:1–85.
Article9. Krecke KN, Gisvold JJ. Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am J Roentgenol. 1993; 161:957–960.
Article10. Yeatman TJ, Cantor AB, Smith TJ, Smith SK, Reintgen DS, Miller MS, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg. 1995; 222:549–559. discussion 559-61.11. du Toit RS, Locker AP, Ellis IO, Elston CW, Nicholson RI, Blamey RW. Invasive lobular carcinomas of the breast--the prognosis of histopathological subtypes. Br J Cancer. 1989; 60:605–609.
Article12. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996; 77:113–120.
Article13. Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW, Stewart HJ. Infiltrating lobular carcinoma of the breast: an evaluation of the incidence and consequence of bilateral disease. Br J Surg. 1983; 70:513–516.
Article14. Toikkanen S, Pylkkänen L, Joensuu H. Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Cancer. 1997; 76:1234–1240.
Article15. Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005; 23:41–48.
Article16. Yu J, Dabbs DJ, Shuai Y, Niemeier LA, Bhargava R. Classical-type invasive lobular carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor characteristics. Am J Clin Pathol. 2011; 136:88–97.
Article17. King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011; 260:50–60.
Article18. Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am. 2007; 45:863–880.
Article19. Imamura T, Isomoto I, Sueyoshi E, Yano H, Uga T, Abe K, et al. Diagnostic performance of ADC for Non-mass-like breast lesions on MR imaging. Magn Reson Med Sci. 2010; 9:217–225.
Article20. Cheng L, Bai Y, Zhang J, Liu M, Li X. Difference of apparent diffusion coefficient in breast mass and non-mass like enhancement lesions. Proc Intl Soc Mag Reson Med. 2011; 19:3084.21. Woodhams R, Ramadan S, Stanwell P, Sakamoto S, Hata H, Ozaki M, et al. Diffusion-weighted imaging of the breast: principles and clinical applications. Radiographics. 2011; 31:1059–1084.
Article22. Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010; 23:619–623.
Article23. Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008; 7:23–29.
Article24. Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the Apparent Diffusion Coefficient (ADC) values for breast tumors. Korean J Radiol. 2007; 8:390–396.
Article25. Tzias D, O'flynn E, Allen S, Wilson R. Apparent diffusion coefficient values from diffusion weighted imaging for invasive lobular cancer of the breast. ECR. 2013; DOI: 10.1594/ecr2013/C-0987.26. Choi BB, Kim SH, Kang BJ, Lee JH, Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012; 10:126.
Article27. Ludovini V, Sidoni A, Pistola L, Bellezza G, De Angelis V, Gori S, et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res Treat. 2003; 81:159–168.
Article28. Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001; 42:9–16.29. Kumar R, Lal N, Alavi A. 18F-FDG PET in detecting primary breast cancer. J Nucl Med. 2007; 48:1751. author reply 1752.
Article30. Cho GY, Moy L, DeGregorio S, Kim S, Moccaldi M, Kwon J, et al. Background Parenchymal Enhancement (BPE) in healthy subjects and breast cancer patients: a quantitative evaluation and comparison with diffusion-weighted imaging. Paper presented at: 20th Scientific Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM). 2012 May 5-11; Melbourne: Australia;p. 0448.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation Between Apparent Diffusion Coefficients and Standardized Uptake Values in Hybrid 18F-FDG PET/MR: Preliminary Results in Rectal Cancer
- Diagnostic Value of SUV in 18F-FDG PET/CT for Papillary Thyroid Cancer
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer
- Can Initial 18F-FDG PET-CT Imaging Give Information onMetastasis in Patients with Primary Renal Cell Carcinoma?
- Clinical usefulness of FDG-PET in patients with hepatocellular carcinoma undergoing surgical resection