Chonnam Med J.
2017 May;53(2):83-94. 10.4068/cmj.2017.53.2.83.
NIRF Heptamethine Cyanine Dye Nanocomplexes for Multi Modal Theranosis of Tumors
- Affiliations
-
- 1Department of Radiology, Chonnam National University Hwasun Hospital, Molecular Theranostics Laboratory, Hwasun, Korea. yjeong@jnu.ac.kr
- KMID: 2379280
- DOI: http://doi.org/10.4068/cmj.2017.53.2.83
Abstract
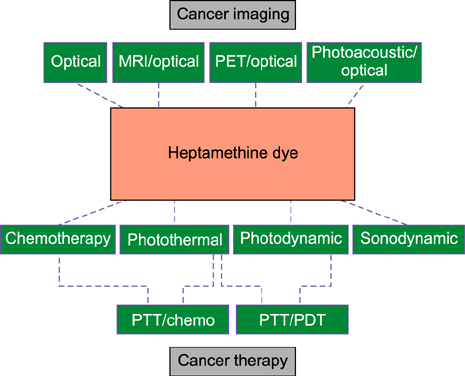
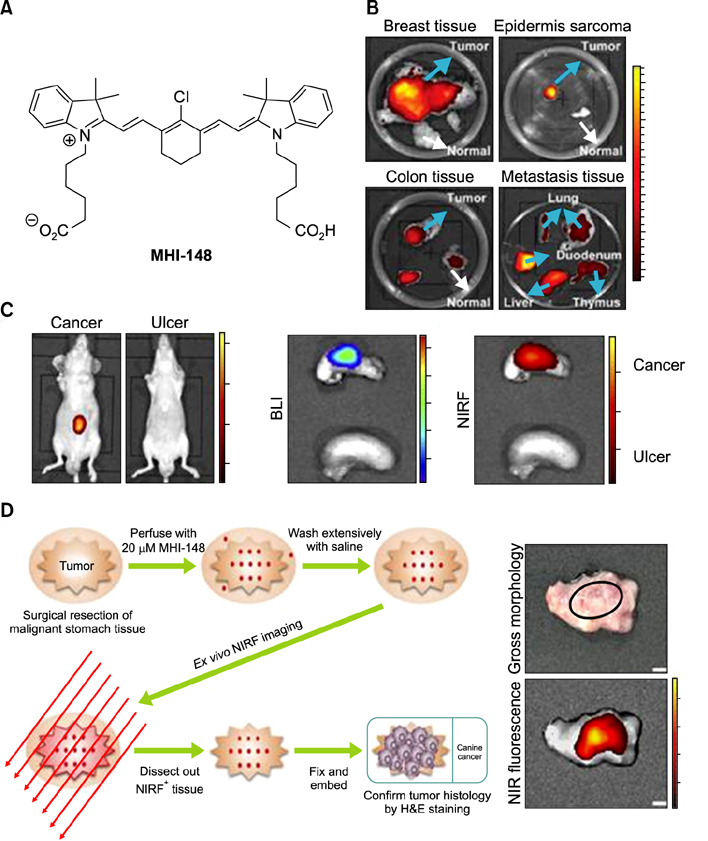
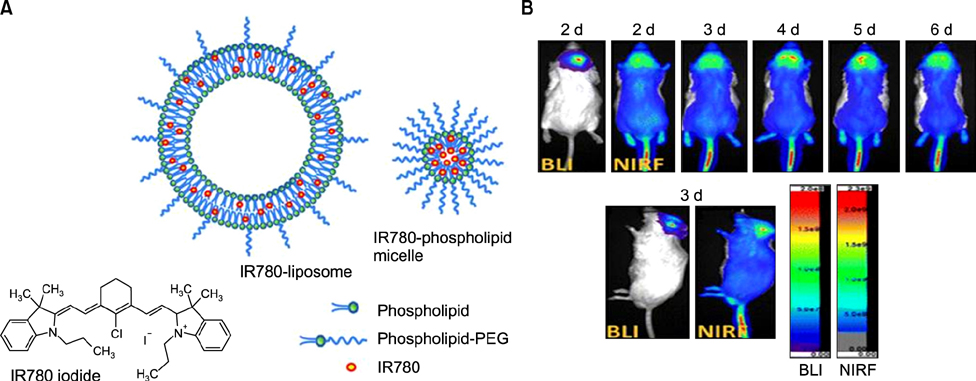
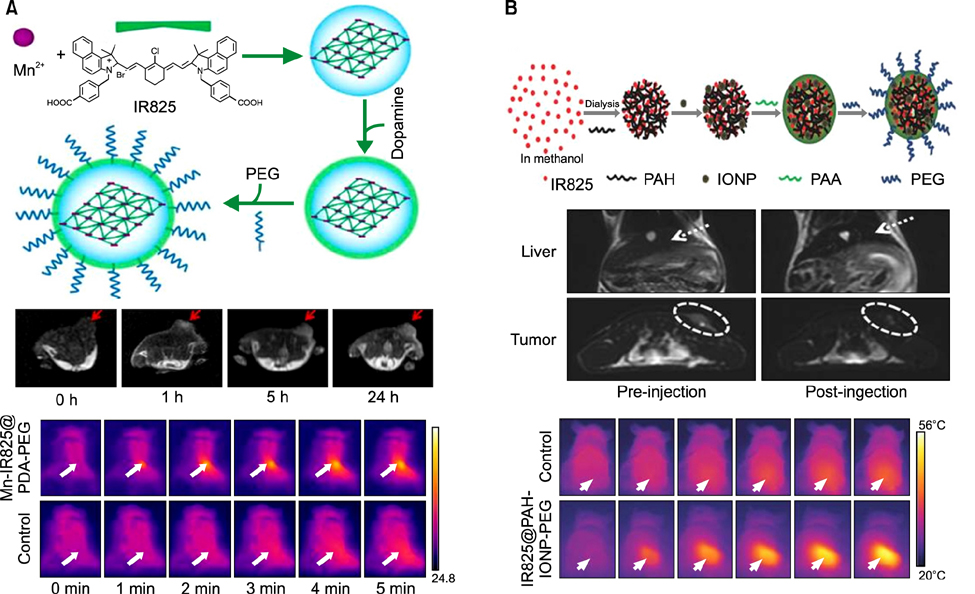
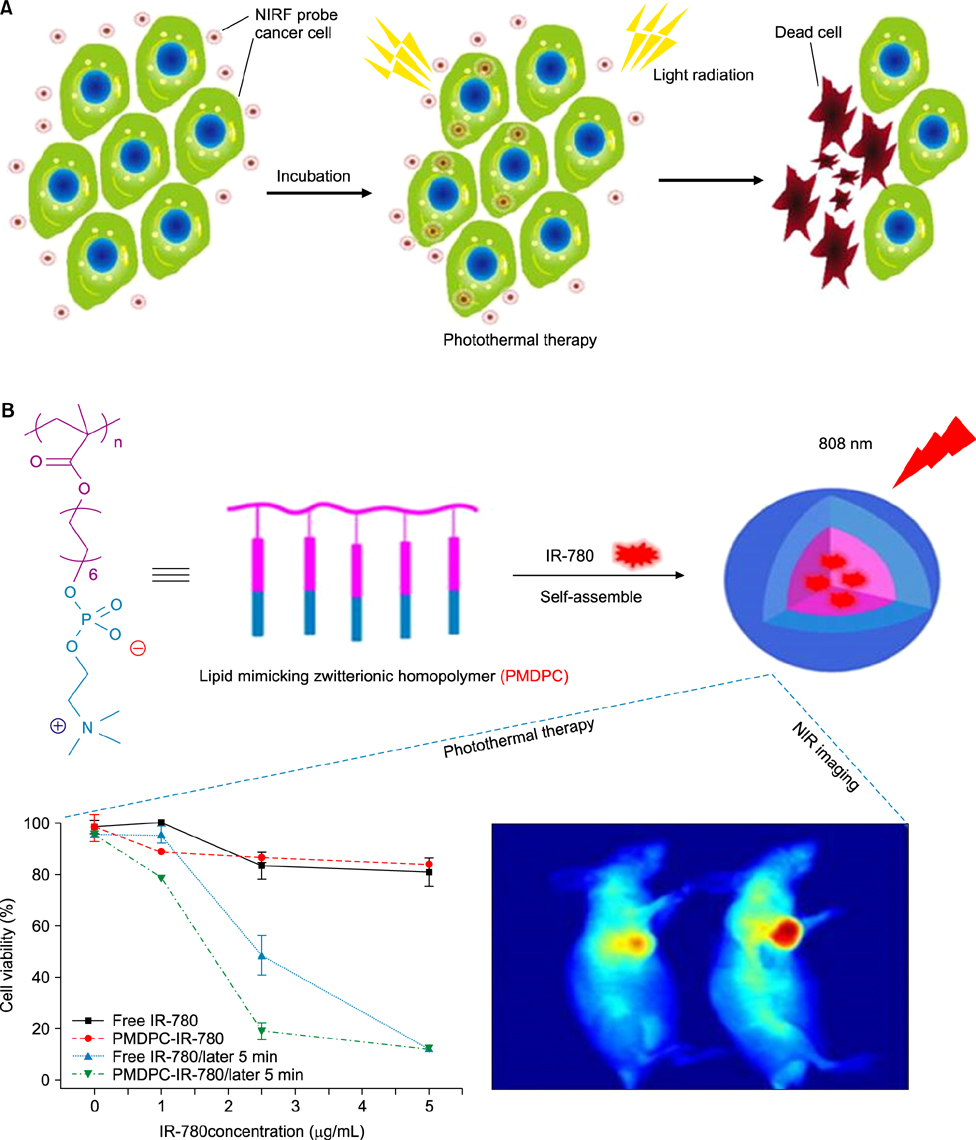
- Heptamethine cyanine dyes are categorized as a class of near infrared fluorescent (NIRF) dyes which have been discovered to have tumor targeting and accumulation capability. This unique feature of NIRF dye makes it a promising candidate for imaging, targeted therapy and also as a drug delivery vehicle for various types of cancers. The favored uptake of dyes only in cancer cells is facilitated by several factors which include organic anion-transporting polypeptides, high mitochondrial membrane potential and tumor hypoxia in cancer cells. Currently nanotechnology has opened possibilities for multimodal or multifunctional strategies for cancer treatment. Including heptamethine cyanine dyes in nanoparticle based delivery systems have generally improved its theranostic ability by several fold owing to the multiple functionalities and structural features of heptamethine dyes. For this reason, nanocomplexes with NIRF heptamethine cyanine dye probe are preferred over non-targeting dyes such as indo cyanine green (ICG). This review sums up current trends and progress in NIRF heptamethine cyanine dye, including dye properties, multifunctional imaging and therapeutic applications in cancer.
MeSH Terms
Figure
Reference
-
1. Jian WH, Yu TW, Chen CJ, Huang WC, Chiu HC, Chiang WH. Indocyanine green-encapsulated hybrid polymeric nanomicelles for photothermal cancer therapy. Langmuir. 2015; 31:6202–6210.
Article2. Ma Y, Tong S, Bao G, Gao C, Dai Z. Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy. Biomaterials. 2013; 34:7706–7714.
Article3. Miki K, Inoue T, Kobayashi Y, Nakano K, Matsuoka H, Yamauchi F, et al. Near-infrared dye-conjugated amphiphilic hyaluronic acid derivatives as a dual contrast agent for in vivo optical and photoacoustic tumor imaging. Biomacromolecules. 2015; 16:219–227.
Article4. Wang H, Agarwal P, Zhao S, Yu J, Lu X, He X. A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. Nat Commun. 2015; 6:10081.
Article5. Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J Pharm Sci. 2013; 102:6–28.
Article6. Zhao P, Zheng M, Luo Z, Gong P, Gao G, Sheng Z, et al. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Sci Rep. 2015; 5:14258.
Article7. Zhao P, Zheng M, Yue C, Luo Z, Gong P, Gao G, et al. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials. 2014; 35:6037–6046.
Article8. Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011; 8:447–456.
Article9. Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016; 10:2436–2446.
Article10. GhavamiNejad A, SamariKhalaj M, Aguilar LE, Park CH, Kim CS. pH/NIR light-controlled multidrug release via a mussel-inspired nanocomposite hydrogel for chemo-photothermal cancer therapy. Sci Rep. 2016; 6:33594.
Article11. Jung HS, Han J, Lee JH, Lee JH, Choi JM, Kweon HS, et al. Enhanced NIR radiation-triggered hyperthermia by mitochondrial targeting. J Am Chem Soc. 2015; 137:3017–3023.
Article12. Kim J, Oh J, Kang HW, Feldman MD, Milner TE. Photothermal response of superparamagnetic iron oxide nanoparticles. Lasers Surg Med. 2008; 40:415–421.
Article13. Wang T, Wang D, Yu H, Wang M, Liu J, Feng B, et al. Intracellularly acid-switchable multifunctional micelles for combinational photo/chemotherapy of the drug-resistant tumor. ACS Nano. 2016; 10:3496–3508.
Article14. Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q, et al. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials. 2016; 81:114–124.
Article15. Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, et al. Biomineralization-Inspired Synthesis of Copper Sulfide-Ferritin Nanocages as Cancer Theranostics. ACS Nano. 2016; 10:3453–3460.
Article16. Wu M, Zhang D, Zeng Y, Wu L, Liu X, Liu J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology. 2015; 26:115102.
Article17. Zhang N, Xu X, Zhang X, Qu D, Xue L, Mo R, et al. Nanocomposite hydrogel incorporating gold nanorods and paclitaxel-loaded chitosan micelles for combination photothermal-chemotherapy. Int J Pharm. 2016; 497:210–221.
Article18. Zhou F, Wu S, Wu B, Chen WR, Xing D. Mitochondria-targeting single-walled carbon nanotubes for cancer photothermal therapy. Small. 2011; 7:2727–2735.
Article19. Zhou Z, Sun Y, Shen J, Wei J, Yu C, Kong B, et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials. 2014; 35:7470–7478.
Article20. Shi C, Wu JB, Pan D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J Biomed Opt. 2016; 21:50901.
Article21. Yang X, Shao C, Wang R, Chu CY, Hu P, Master V, et al. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J Urol. 2013; 189:702–710.
Article22. Yang X, Shi C, Tong R, Qian W, Zhau HE, Wang R, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res. 2010; 16:2833–2844.
Article23. Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011; 32:7127–7138.
Article24. Yuan J, Yi X, Yan F, Wang F, Qin W, Wu G, et al. Near infrared fluorescence imaging of prostate cancer using heptamethine carbocyanine dyes. Mol Med Rep. 2015; 11:821–828.
Article25. Shi C, Wu JB, Chu GC, Li Q, Wang R, Zhang C, et al. Heptamethine carbocyanine dye-mediated near-infrared imaging of canine and human cancers through the HIF-1α/OATPs signaling axis. Oncotarget. 2014; 5:10114–10126.
Article26. Conceicao DS, Ferreira DP, Ferreira LF. Photochemistry and cytotoxicity evaluation of heptamethinecyanine Near Infrared (NIR) dyes. Int J Mol Sci. 2013; 14:18557–18571.
Article27. Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X, et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials. 2010; 31:6612–6617.
Article28. Wang Y, Liu T, Zhang E, Luo S, Tan X, Shi C. Preferential accumulation of the near infrared heptamethine dye IR-780 in the mitochondria of drug-resistant lung cancer cells. Biomaterials. 2014; 35:4116–4124.
Article29. Wu JB, Shao C, Li X, Shi C, Li Q, Hu P, et al. Near-infrared fluorescence imaging of cancer mediated by tumor hypoxia and HIF1α/OATPs signaling axis. Biomaterials. 2014; 35:8175–8185.
Article30. Zhao N, Zhang C, Zhao Y, Bai B, An J, Zhang H, et al. Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget. 2016; 7:57277–57289.
Article31. Zhang E, Luo S, Tan X, Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials. 2014; 35:771–778.
Article32. Buxhofer-Ausch V, Secky L, Wlcek K, Svoboda M, Kounnis V, Briasoulis E, et al. Tumor-specific expression of organic anion-transporting polypeptides: transporters as novel targets for cancer therapy. J Drug Deliv. 2013; 2013:863539.33. Singh AK, Hahn MA, Gutwein LG, Rule MC, Knapik JA, Moudgil BM, et al. Multi-dye theranostic nanoparticle platform for bioimaging and cancer therapy. Int J Nanomedicine. 2012; 7:2739–2750.34. Corem-Salkmon E, Perlstein B, Margel S. Design of near-infrared fluorescent bioactive conjugated functional iron oxide nanoparticles for optical detection of colon cancer. Int J Nanomedicine. 2012; 7:5517–5527.35. Li S, Johnson J, Peck A, Xie Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J Transl Med. 2017; 15:18.
Article36. Lu C, Das S, Magut PK, Li M, El-Zahab B, Warner IM. Irradiation induced fluorescence enhancement in PEGylated cyanine-based NIR nano- and mesoscale GUMBOS. Langmuir. 2012; 28:14415–14423.
Article37. Yang Y, Liu J, Liang C, Feng L, Fu T, Dong Z, et al. Nanoscale metal-organic particles with rapid clearance for magnetic resonance imaging-guided photothermal therapy. ACS Nano. 2016; 10:2774–2781.
Article38. Yeh CS, Su CH, Ho WY, Huang CC, Chang JC, Chien YH, et al. Tumor targeting and MR imaging with lipophilic cyanine-mediated near-infrared responsive porous Gd silicate nanoparticles. Biomaterials. 2013; 34:5677–5688.
Article39. Chen Q, Liang C, Wang X, He J, Li Y, Liu Z. An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 2014; 35:9355–9362.
Article40. Xiao L, Zhang Y, Yue W, Xie X, Wang JP, Chordia MD, et al. Heptamethine cyanine based (64)Cu-PET probe PC-1001 for cancer imaging: synthesis and in vivo evaluation. Nucl Med Biol. 2013; 40:351–360.
Article41. Song X, Gong H, Liu T, Cheng L, Wang C, Sun X, et al. J-aggregates of organic dye molecules complexed with iron oxide nanoparticles for imaging-guided photothermal therapy under 915-nm light. Small. 2014; 10:4362–4370.
Article42. Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Methods. 2016; 13:639–650.
Article43. Shi S, Liu Y, Chen Y, Zhang Z, Ding Y, Wu Z, et al. Versatile pH-response micelles with high cell-penetrating helical diblock copolymers for photoacoustic imaging guided synergistic chemo-photothermal therapy. Theranostics. 2016; 6:2170–2182.
Article44. Okuda T, Kobayashi Y, Yanamoto S, Okamoto H. PEG conjugation of a near-infrared fluorescent probe for noninvasive dual imaging of lung deposition and gene expression by pulmonary gene delivery. J Drug Target. 2012; 20:801–812.
Article45. Costantini P, Jacotot E, Decaudin D, Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000; 92:1042–1053.
Article46. Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010; 9:447–464.
Article47. Zhang E, Zhang C, Su Y, Cheng T, Shi C. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug Discov Today. 2011; 16:140–146.
Article48. Wu JB, Shi C, Chu GC, Xu Q, Zhang Y, Li Q, et al. Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials. 2015; 67:1–10.
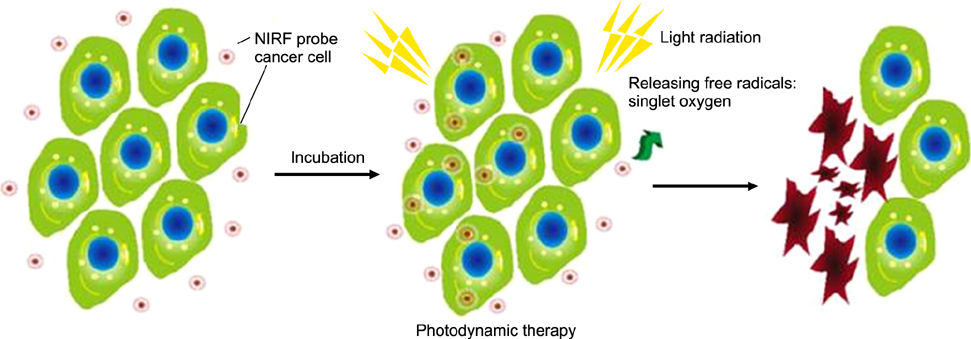
Article49. Chen Y, Li Z, Wang H, Wang Y, Han H, Jin Q, et al. IR-780 loaded phospholipid mimicking homopolymeric micelles for near-IR imaging and photothermal therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2016; 8:6852–6858.
Article50. Lin T, Yuan A, Zhao X, Lian H, Zhuang J, Chen W, et al. Self-assembled tumor-targeting hyaluronic acid nanoparticles for photothermal ablation in orthotopic bladder cancer. Acta Biomater. 2017; DOI: 10.1016/j.actbio.2017.02.021. [Epub ahead of print].
Article51. Cheng L, He W, Gong H, Wang C, Chen Q, Cheng Z, et al. PEGylated micelle nanoparticles encapsulating a non-fluorescent near-infrared organic dye as a safe and highly-effective photothermal agent for in vivo cancer therapy. Adv Funct Mater. 2013; 23:5893–5902.
Article52. Yuan A, Qiu X, Tang X, Liu W, Wu J, Hu Y. Self-assembled PEG-IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials. 2015; 51:184–193.
Article53. Yi X, Wang F, Qin W, Yang X, Yuan J. Near-infrared fluorescent probes in cancer imaging and therapy: an emerging field. Int J Nanomedicine. 2014; 9:1347–1365.
Article54. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61:250–281.
Article55. Felsher DW. Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer. 2003; 3:375–380.
Article56. Tan X, Luo S, Wang D, Su Y, Cheng T, Shi C. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials. 2012; 33:2230–2239.
Article57. Luo S, Tan X, Qi Q, Guo Q, Ran X, Zhang L, et al. A multifunctional heptamethine near-infrared dye for cancer theranosis. Biomaterials. 2013; 34:2244–2251.
Article58. Pais-Silva C, de Melo-Diogo D, Correia IJ. IR780-loaded TPGSTOS micelles for breast cancer photodynamic therapy. Eur J Pharm Biopharm. 2017; 113:108–117.
Article59. Wan GY, Liu Y, Chen BW, Liu YY, Wang YS, Zhang N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016; 13:325–338.
Article60. Li Y, Zhou Q, Deng Z, Pan M, Liu X, Wu J, et al. IR-780 dye as a sonosensitizer for sonodynamic therapy of breast tumor. Sci Rep. 2016; 6:25968.
Article61. Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, et al. Single-step assembly of DOX/ICG loaded lipid--polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano. 2013; 7:2056–2067.
Article62. Guo F, Yu M, Wang J, Tan F, Li N. Smart IR780 theranostic nanocarrier for tumor-specific therapy: hyperthermia-mediated bubble-generating and folate-targeted liposomes. ACS Appl Mater Interfaces. 2015; 7:20556–20567.
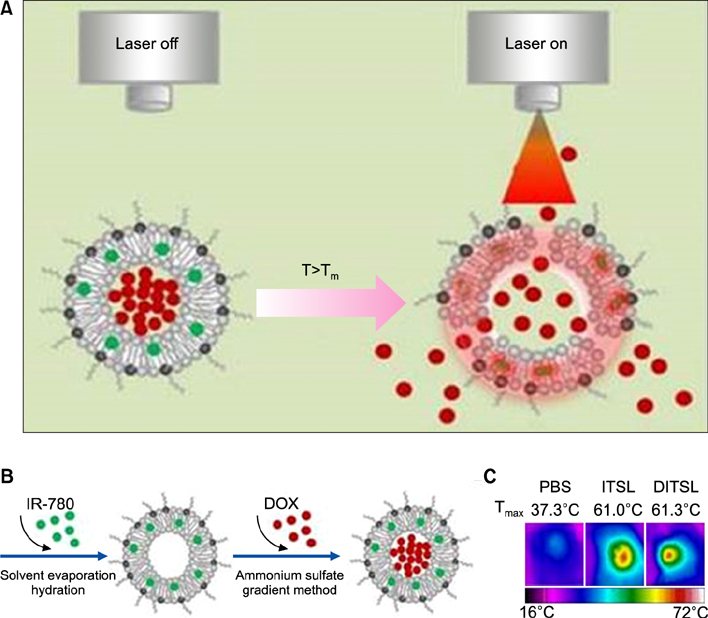
Article63. Yan F, Duan W, Li Y, Wu H, Zhou Y, Pan M, et al. NIR-laser-controlled drug release from DOX/IR-780-loaded temperature-sensitive-liposomes for chemo-photothermal synergistic tumor therapy. Theranostics. 2016; 6:2337–2351.
Article64. Peng CL, Chen YI, Liu HJ, Lee PC, Luo TY, Shieh MJ. A novel temperature-responsive micelle for enhancing combination therapy. Int J Nanomedicine. 2016; 11:3357–3369.65. Guo F, Yu M, Wang J, Tan F, Li N. The mitochondria-targeted and IR780-regulated theranosomes for imaging and enhanced photodynamic/photothermal therapy. RSC Adv. 2016; 6:11070–11076.
Article66. Jiang C, Cheng H, Yuan A, Tang X, Wu J, Hu Y. Hydrophobic IR780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomater. 2015; 14:61–69.
Article67. Luo S, Tan X, Fang S, Wang Y, Liu T, Wang X, et al. Mitochondria-targeted small-molecule fluorophores for dual modal cancer phototherapy. Adv Funct Mater. 2016; 26:2826–2835.
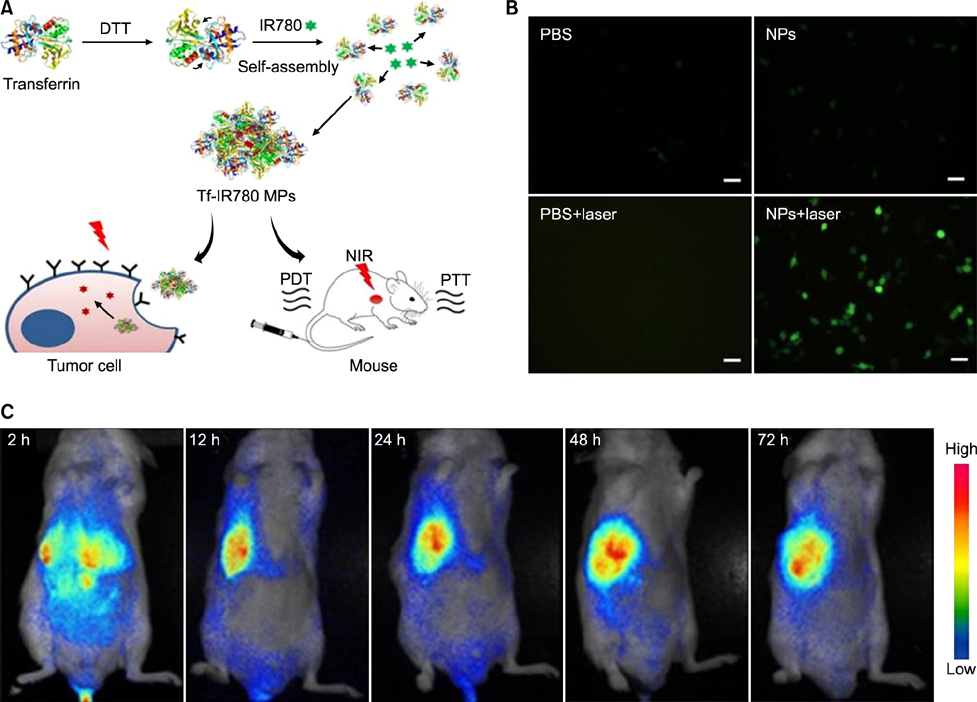
Article68. Wang K, Zhang Y, Wang J, Yuan A, Sun M, Wu J, et al. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci Rep. 2016; 6:27421.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fluorescence Quenching Causes Systematic Dye Bias in Microarray Experiments Using Cyanine Dye
- The effect of near-infrared fluorescence conjugation on the anti-cancer potential of cetuximab
- Assessment of Collagen-Induced Arthritis Using Cyanine 5.5 Conjugated with Hydrophobically Modified Glycol Chitosan Nanoparticles: Correlation with 18F-Fluorodeoxyglucose Positron Emission Tomography Data
- Effect of Multi-modal Interventions for Smoking Cessation in a University Setting: A Short Course of Varenicline, Financial Incentives, E-mail and Short Message Service
- Current Status of PSMA‑Targeted Radioligand Therapy in the Era of Radiopharmaceutical Therapy Acquiring Marketing Authorization