Obstet Gynecol Sci.
2017 May;60(3):274-282. 10.5468/ogs.2017.60.3.274.
Serum from pregnant women with gestational diabetes mellitus increases the expression of FABP4 mRNA in primary subcutaneous human pre-adipocytes
- Affiliations
-
- 1Institute of Medical Science, Kangwon National University School of Medicine, Chuncheon, Korea. rapidhwang@kangwon.ac.kr
- 2Department of Obstetrics and Gynecology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3Department of Obstetrics and Gynecology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2378589
- DOI: http://doi.org/10.5468/ogs.2017.60.3.274
Abstract
OBJECTIVE
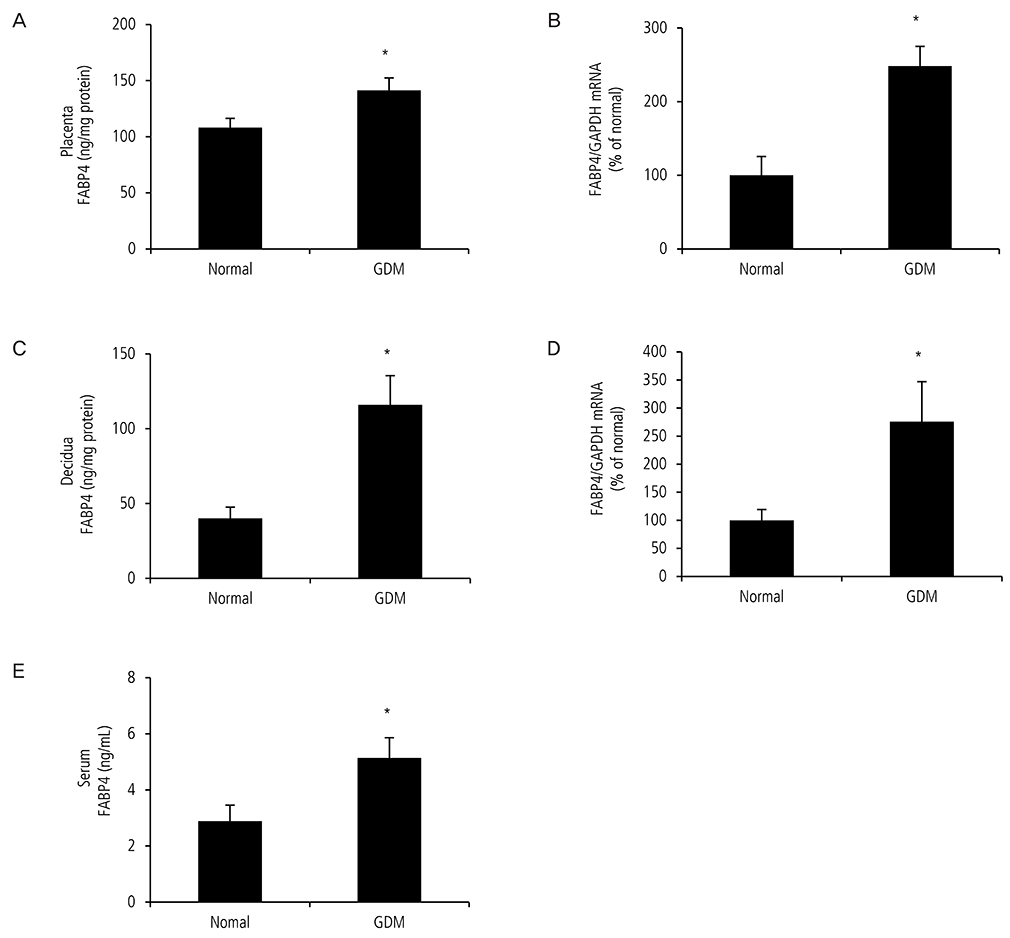
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy. It can result in pregnancy complications such as birth injury, stillbirth. Fatty acid-binding protein 4 (FABP4), found in adipose tissue, is associated with insulin resistance, and type 2 diabetes. The aim of this study was to investigate whether FABP4 in the placenta and decidua of pregnant women with GDM is higher than that in normal pregnant women, and whether serum from pregnant women with GDM may cause adipocytes to secrete more FABP4 than does serum from a normal pregnant group.
METHODS
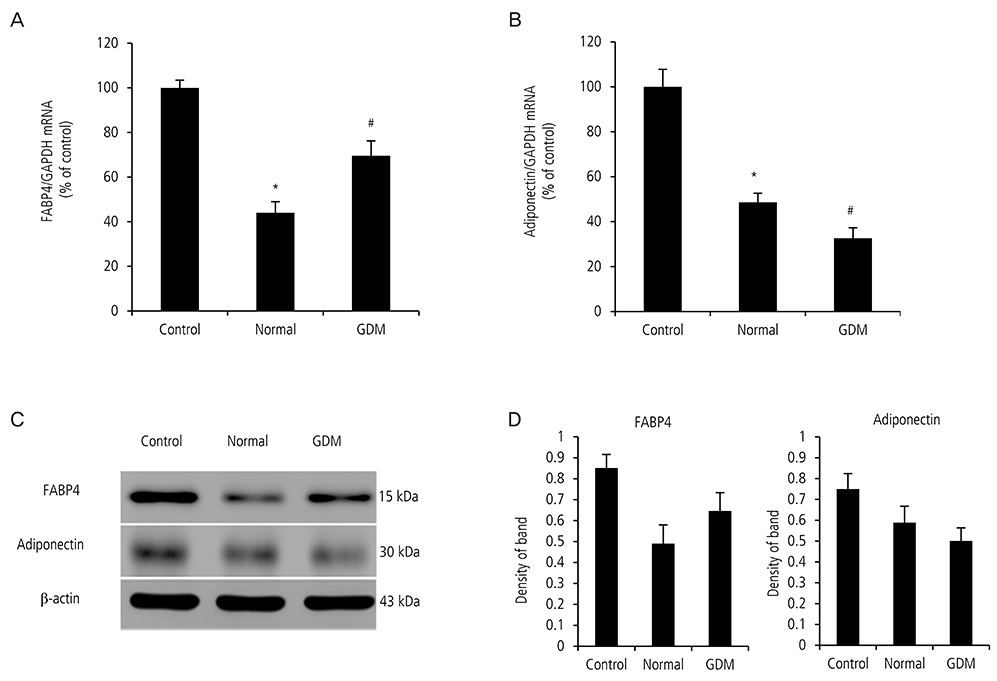
We obtained placentas, deciduas, and serum from 12 pregnant women with GDM and 12 normal pregnant women and performed enzyme-linked immunosorbent assay, real time quantitative-polymerase chain reaction. We cultured human pre-adipocytes for 17 days with GDM and non-GDM serum and performed western blot, real time quantitative-polymerase chain reaction, and oil red O staining.
RESULTS
Expression of FABP4 in serum, placenta and decidua of pregnant women with GDM was significantly higher than that in normal pregnant women. Serum from pregnant women with GDM increased the expression of FABP4 mRNA and decreased the expression of adiponectin mRNA in human pre-adipocytes significantly. Adipocyte cultured in GDM serum showed significantly greater lipid accumulation than those cultured in normal serum.
CONCLUSION
Our results suggest that FABP4 is higher in placenta and decidua from pregnant women with GDM. Increased circulating FABP4 in maternal serum from pregnant women with GDM may originate from adipocytes and the placenta. Circulating FABP4 can induce increased insulin resistance and decreased insulin sensitivity.
MeSH Terms
-
Adipocytes
Adiponectin
Adipose Tissue
Birth Injuries
Blotting, Western
Decidua
Diabetes, Gestational*
Enzyme-Linked Immunosorbent Assay
Female
Glucose Intolerance
Humans
Humans*
Insulin Resistance
Placenta
Pregnancy
Pregnancy Complications
Pregnancy in Diabetics
Pregnant Women*
RNA, Messenger*
Stillbirth
Adiponectin
RNA, Messenger
Figure
Reference
-
1. Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus: the insulin and cytokine network. Diabetes Care. 2007; 30:Suppl 2. S120–S126.2. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005; 115:485–491.3. Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008; 57:606–613.4. Kalkhoff RK, Kissebah AH, Kim HJ. Carbohydrate and lipid metabolism during normal pregnancy: relationship to gestational hormone action. Semin Perinatol. 1978; 2:291–307.5. Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988; 67:341–347.6. Waters TP, Schultz BA, Mercer BM, Catalano PM. Effect of 17alpha-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol. 2009; 114:45–49.7. Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986; 83:3786–3790.8. Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty acid-binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2015; 8:Suppl 3. 23–33.9. Toruner F, Altinova AE, Akturk M, Kaya M, Arslan E, Bukan N, et al. The relationship between adipocyte fatty acid binding protein-4, retinol binding protein-4 levels and early diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011; 91:203–207.10. Li YY, Xiao R, Li CP, Huangfu J, Mao JF. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med Sci Monit. 2015; 21:426–431.11. Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006; 49:1677–1685.12. Hauguel-de Mouzon S, Catalano P. Adiponectin: are measurements clinically useful in pregnancy? Diabetes Care. 2013; 36:1434–1436.13. Mather KJ, Goldberg RB. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab. 2014; 28:107–117.14. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007; 30:Suppl 2. S251–S260.15. Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007; 447:959–965.16. Buchanan TA, Metzger BE, Freinkel N, Bergman RN. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990; 162:1008–1014.17. Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001; 286:2516–2518.18. Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011; 18:409–416.19. Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: gestational diabetes mellitus. Definition, aetiological and clinical aspects. Eur J Endocrinol. 2016; 174:R43–R51.20. Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001; 86:568–573.21. Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004; 27:799–800.22. Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004; 89:2306–2311.