Ann Dermatol.
2017 Jun;29(3):268-275. 10.5021/ad.2017.29.3.268.
Periostin in Mature Stage Localized Scleroderma
- Affiliations
-
- 1Department of Dermatology, SMG-SNU Boramae Medical Center, Seoul, Korea. snuhdm@gmail.com
- 2Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 3Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea.
- KMID: 2378524
- DOI: http://doi.org/10.5021/ad.2017.29.3.268
Abstract
- BACKGROUND
Periostin is a novel matricellular protein expressed in many tissues, including bone, periodontal ligament, and skin. Although its expression is prominent in various fibrotic conditions, studies of periostin in localized scleroderma are rare.
OBJECTIVE
To investigate the expression of periostin and other molecules in localized scleroderma.
METHODS
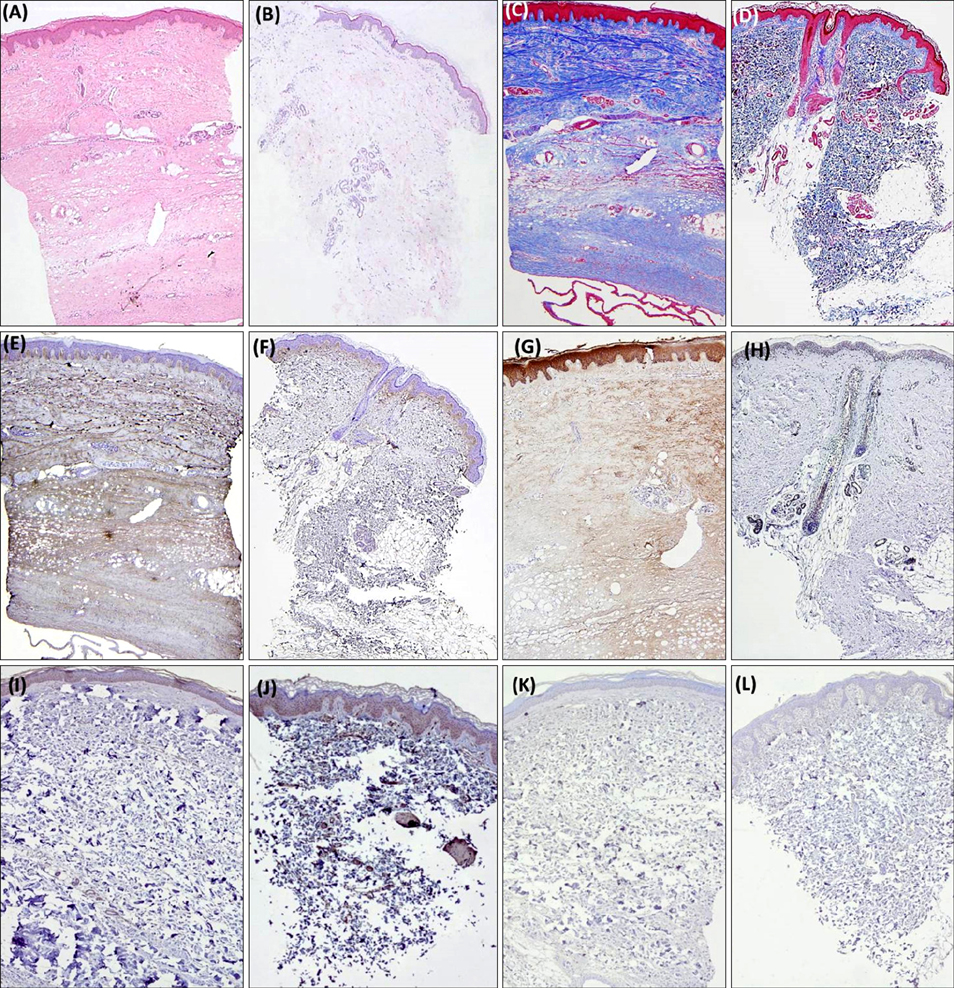
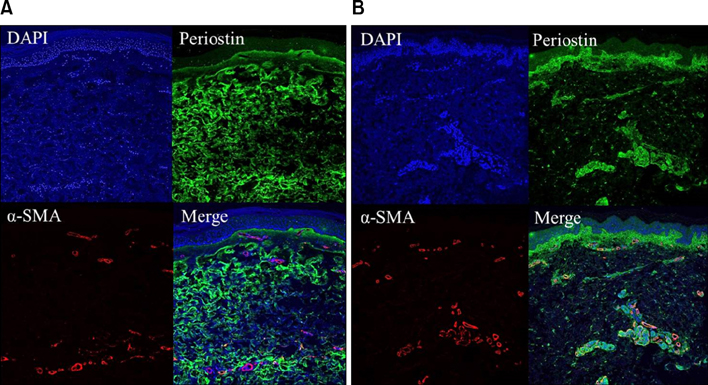
A retrospective study of 14 patients with confirmed mature stage localized scleroderma was undertaken. Fourteen age-matched and biopsy site-matched subjects with normal skin were included as controls. Collagen fiber deposition, periostin, procollagen, transforming growth factor-β, and matrix metalloproteinase (MMP)-1 expression were assessed and compared between the two groups. Co-localization of α-smooth muscle actin and periostin was evaluated using confocal microscopy.
RESULTS
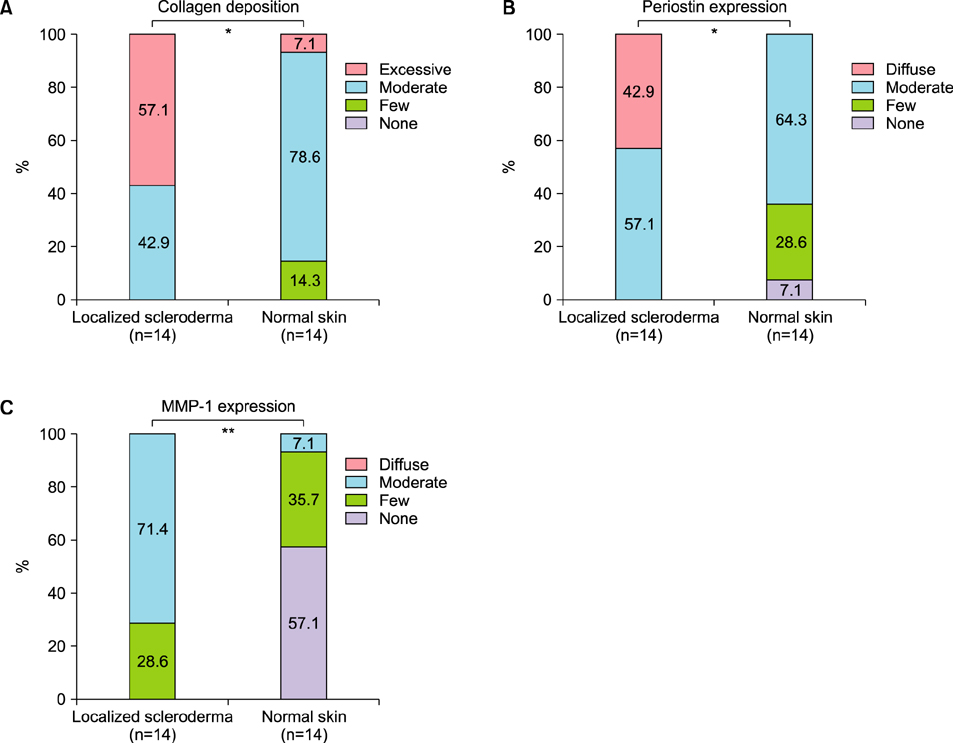
Periostin was predominantly expressed along the dermo-epidermal junction in the controls. Conversely, patients with localized scleroderma demonstrated increased collagen fiber deposition and periostin expression that was more widely distributed along the entire dermis. MMP-1 staining showed increased expression in the epidermis and dermis of patients compared to scanty expression in the controls. A semi-quantitative evaluation showed a higher proportion of excessive collagen bundle deposition (57.1% vs. 7.1%, p=0.013), diffuse periostin positivity (42.9% vs. 0%, p=0.016), and moderate MMP-1 positivity (71.4% vs. 7.1%, p=0.001) in patients than in the controls.
CONCLUSION
Compared to the controls, patients with localized scleroderma had enhanced periostin expression corresponding to increased collagen fiber deposition and unexpected overexpression of MMP-1. The results of this human in vivo study may implicate the pathogenesis of localized scleroderma.
Keyword
MeSH Terms
Figure
Reference
-
1. Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993; 294:271–278.
Article2. Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, et al. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 2004; 229:857–868.
Article3. Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008; 2:9–17.
Article4. Oku E, Kanaji T, Takata Y, Oshima K, Seki R, Morishige S, et al. Periostin and bone marrow fibrosis. Int J Hematol. 2008; 88:57–63.
Article5. Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994; 103:359–363.
Article6. Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006; 126:1761–1769.
Article7. Sato S, Hayakawa I, Hasegawa M, Fujimoto M, Takehara K. Function blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosis. J Invest Dermatol. 2003; 120:542–547.
Article8. Tomimura S, Ogawa F, Iwata Y, Komura K, Hara T, Muroi E, et al. Autoantibodies against matrix metalloproteinase-1 in patients with localized scleroderma. J Dermatol Sci. 2008; 52:47–54.
Article9. Hasegawa M, Sato S, Takehara K. Augmented production of transforming growth factor-beta by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Arch Dermatol Res. 2004; 296:89–93.
Article10. Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford). 2008; 47:Suppl 5. v8–v9.
Article11. Kuroda K, Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res. 1997; 289:567–572.
Article12. Yamaguchi Y, Ono J, Masuoka M, Ohta S, Izuhara K, Ikezawa Z, et al. Serum periostin levels are correlated with progressive skin sclerosis in patients with systemic sclerosis. Br J Dermatol. 2013; 168:717–725.
Article13. Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012; 7:e41994.
Article14. Park HR, Min SK, Cho HD, Kim KH, Shin HS, Park YE. Expression profiles of p63, p53, survivin, and hTERT in skin tumors. J Cutan Pathol. 2004; 31:544–549.
Article15. Jimenez SA, Hitraya E, Varga J. Pathogenesis of scleroderma. Collagen. Rheum Dis Clin North Am. 1996; 22:647–674.16. Vuorio T, Mäkelä JK, Kähäri VM, Vuorio E. Coordinated regulation of type I and type III collagen production and mRNA levels of pro alpha 1(I) and pro alpha 2(I) collagen in cultured morphea fibroblasts. Arch Dermatol Res. 1987; 279:154–160.
Article17. Kähäri VM, Sandberg M, Kalimo H, Vuorio T, Vuorio E. Identification of fibroblasts responsible for increased collagen production in localized scleroderma by in situ hybridization. J Invest Dermatol. 1988; 90:664–670.
Article18. Krieg T, Braun-Falco O, Perlish JS, Fleischmajer R. Collagen synthesis in generalized morphea. Arch Dermatol Res. 1983; 275:393–396.
Article19. Sato S, Ihn H, Soma Y, Igarashi A, Tamaki T, Kikuchi K, et al. Antihistone antibodies in patients with localized scleroderma. Arthritis Rheum. 1993; 36:1137–1141.
Article20. Nagai M, Hasegawa M, Takehara K, Sato S. Novel autoantibody to Cu/Zn superoxide dismutase in patients with localized scleroderma. J Invest Dermatol. 2004; 122:594–601.
Article21. Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am. 1996; 22:709–735.
Article22. Takehara K. Hypothesis: pathogenesis of systemic sclerosis. J Rheumatol. 2003; 30:755–759.23. Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006; 168:499–510.
Article24. Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7-Smurf-mediated negative regulation of TGF-beta signaling in scleroderma fibroblasts. J Clin Invest. 2004; 113:253–264.
Article25. Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007; 56:334–344.
Article26. Wu M, Varga J. In perspective: murine models of scleroderma. Curr Rheumatol Rep. 2008; 10:173–182.
Article27. Kawakami T, Ihn H, Xu W, Smith E, LeRoy C, Trojanowska M. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J Invest Dermatol. 1998; 110:47–51.
Article28. Pannu J, Gore-Hyer E, Yamanaka M, Smith EA, Rubinchik S, Dong JY, et al. An increased transforming growth factor beta receptor type I:type II ratio contributes to elevated collagen protein synthesis that is resistant to inhibition via a kinase-deficient transforming growth factor beta receptor type II in scleroderma. Arthritis Rheum. 2004; 50:1566–1577.
Article29. Pannu J, Gardner H, Shearstone JR, Smith E, Trojanowska M. Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum. 2006; 54:3011–3021.
Article30. Higley H, Persichitte K, Chu S, Waegell W, Vancheeswaran R, Black C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud's phenomenon. Arthritis Rheum. 1994; 37:278–288.
Article31. Farrell AM, Dean D, Charnock M, Wojnarowska F. Distribution of transforming growth factor-beta isoforms TGFbeta 1, TGF-beta 2 and TGF-beta 3 and vascular endothelial growth factor in vulvar lichen sclerosus. J Reprod Med. 2001; 46:117–124.32. Restrepo JF, Guzmán R, Rodríguez G, Iglesias A. Expression of transforming growth factor-beta and platelet-derived growth factor in linear scleroderma. Biomedica. 2003; 23:408–415.
Article33. Dańczak-Pazdrowska A, Kowalczyk MJ, Szramka-Pawlak B, Gornowicz-Porowska J, Szewczyk A, Silny W, et al. Transforming growth factor-β1 in plaque morphea. Postepy Dermatol Alergol. 2013; 30:337–342.
Article34. Querfeld C, Eckes B, Huerkamp C, Krieg T, Sollberg S. Expression of TGF-beta 1, -beta 2 and -beta 3 in localized and systemic scleroderma. J Dermatol Sci. 1999; 21:13–22.35. Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012; 21:331–336.
Article36. Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011; 6:e18410.
Article37. Ro Y, Hamada C, Inaba M, Io H, Kaneko K, Tomino Y. Inhibitory effects of matrix metalloproteinase inhibitor ONO-4817 on morphological alterations in chlorhexidine gluconate-induced peritoneal sclerosis rats. Nephrol Dial Transplant. 2007; 22:2838–2848.
Article38. Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001; 89:201–210.39. Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001; 193:538–545.
Article40. Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994; 370:555–557.
Article41. Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998; 161:3340–3346.42. Narayanan AS, Page RC, Swanson J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-beta in the presence of other inflammatory mediators. Biochem J. 1989; 260:463–469.
Article43. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006; 118:98–104.
Article44. Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007; 101:695–711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical and histopathological survey on scleroderma
- A Case of Localized Scleroderma Improved with Systemic PUVA Therapy
- A Case of Dystrophic Calcinosis Cutis Secondary to Localized Scleroderma
- A Rare Case of Bilateral Frontal Linear Scleroderma (En Coup de Sabre)
- Low-dose PUVA Photochemotherapy in a Patient with Both Localized Scleroderma and Vitiligo