Allergy Asthma Immunol Res.
2017 Jul;9(4):378-382. 10.4168/aair.2017.9.4.378.
Plasma LTE₄/PGF₂α Ratio and Blood Eosinophil Count Are Increased in Elderly Asthmatics With Previous Asthma Exacerbation
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Hallym University School of Medicine, Anyang, Korea.
- 5Department of Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Korea.
- KMID: 2378202
- DOI: http://doi.org/10.4168/aair.2017.9.4.378
Abstract
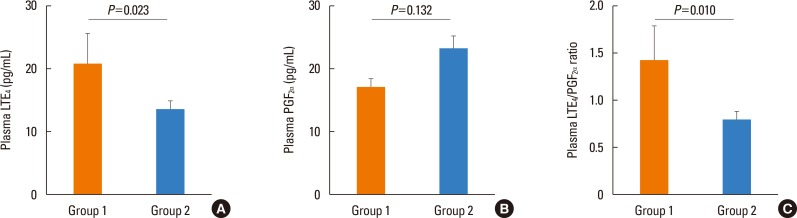
- The tools for asthma control assessment recommended by the current guideline are cognitive function- and effort-dependent, which is substantially impaired in the elderly. The aim of this study is to investigate objective assessment tools of asthma control status and previous asthma exacerbation (AE) in elderly subjects. Asthmatics aged >60 years who were treated with step 2 or 3 by the Global Initiative for Asthma (GINA) guideline were enrolled. During the 12-week study period, the subjects used either 400 µg of budesonide plus 10 mg of montelukast or 800 µg of inhaled budesonide. The occurrence of AE during the 4-week run-in and 12-week treatment period was monitored. After 12-week of treatment, sputum eosinophil count, peripheral eosinophil count, the plasma leukotriene Eâ‚„ (LTEâ‚„), and prostaglandin F₂α (PGF₂α) metabolite levels were measured using the UHPLC/Q-ToF MS system. The study subjects were divided into group 1 (asthmatics who experienced AE during the study period) and group 2 (those who did not). A total of 101 patients aged 60-85 years were enrolled. Twenty-three patients (22.8%) had experienced AE. The plasma LTEâ‚„ level, LTEâ‚„/PGF₂α ratio, and peripheral eosinophil count were significantly higher in group 1 than in group 2 (P=0.023, P=0.010, P=0.033, respectively). The plasma LTEâ‚„/PGF₂α ratio and peripheral eosinophil count at week 12 were significantly associated with previous AE (odds ratio [OR]=1.748, P=0.013; OR=1.256, P=0.027). Receiver operating characteristic (ROC) curves to discriminate the subjects with previous AE, including these 2 parameters, showed that the area under the curve was 0.700 (P=0.004), with 73.9% sensitivity and 47.9% specificity. In conclusion, a combination of plasma LTEâ‚„/PGF₂α ratio and peripheral eosinophil count can be an objective assessment tool which is significantly associated with asthma control status in elderly asthmatics.
Keyword
MeSH Terms
Figure
Reference
-
1. Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, et al. Asthma in the elderly: current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011; 128:S4–S24. PMID: 21872730.
Article2. Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014; 7:8–25. PMID: 25152804.
Article3. Park J, Kim TB, Joo H, Lee JS, Lee SD, Oh YM. Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: a population-based study. Allergy Asthma Immunol Res. 2013; 5:16–25. PMID: 23277874.
Article4. Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015; 7:431–439. PMID: 26122503.
Article5. Song WJ, Jee YK. More effective strategies are needed for elderly asthmatics in real-world practice. Allergy Asthma Immunol Res. 2015; 7:419–420. PMID: 26122501.
Article6. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 (revision) [Internet]. [place unknown]: Global Initiative for Asthma;2016. cited 2016 Oct 6. Available from: http://ginasthma.org/gina-reports.7. Ye YM, Kim SH, Hur GY, Kim JH, Park JW, Shim JJ, et al. Addition of montelukast to low-dose inhaled corticosteroid leads to fewer exacerbations in older patients than medium-dose inhaled corticosteroid monotherapy. Allergy Asthma Immunol Res. 2015; 7:440–448. PMID: 26122504.
Article8. Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017; 47:37–47. PMID: 27533637.
Article9. Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2016; Forthcoming.
Article10. Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014; 6:288–295. PMID: 24991451.
Article11. Sampson AP, Castling DP, Green CP, Price JF. Persistent increase in plasma and urinary leukotrienes after acute asthma. Arch Dis Child. 1995; 73:221–225. PMID: 7492159.
Article12. Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004; 59:100–104. PMID: 14760145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of PAF inhalation on the non-specific bronchial hyperreactivity according to the severity of asthma
- Changes in Plasma and Urine Endothelin Levels During Acute Exacerbation of Asthma
- Circardian Variations of the Peripheral Blood Eosinophil Count and Pulmonary Function in the Bronchial Asthmatics
- Association of Plasma Eotaxin with Asthma Exacerbation and Severity
- Eosinophil cationic protein in relation to bronchial hyperresponsiveness in asthmatic children