Cancer Res Treat.
2017 Apr;49(2):484-493. 10.4143/crt.2016.246.
Young Age Is Associated with Increased Locoregional Recurrence in Node-Positive Breast Cancer with Luminal Subtypes
- Affiliations
-
- 1Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea. chunm@ajou.ac.kr
- 2Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Hematology-Oncology, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- 5Department of Radiation Oncology, Ilsan Paik Hospital, Inje University School of Medicine, Goyang, Korea.
- KMID: 2378121
- DOI: http://doi.org/10.4143/crt.2016.246
Abstract
- PURPOSE
The effects of biological subtypes within breast cancer on prognosis are influenced by age at diagnosis. We investigated the association of young age with locoregional recurrence (LRR) between patients with luminal subtypes versus those with nonluminal subtypes.
MATERIALS AND METHODS
Medical records of 524 breast cancer patients with positive lymph nodes between 1999 and 2010 were reviewed retrospectively. All patients received curative surgery and adjuvant chemotherapy based on contemporary guidelines. Radiation was delivered for patients who underwent breast conserving surgery or those who had four or more positive lymph nodes after mastectomy. Adjuvant hormone therapy was administered to 413 patients with positive hormone receptors according to their menstrual status.
RESULTS
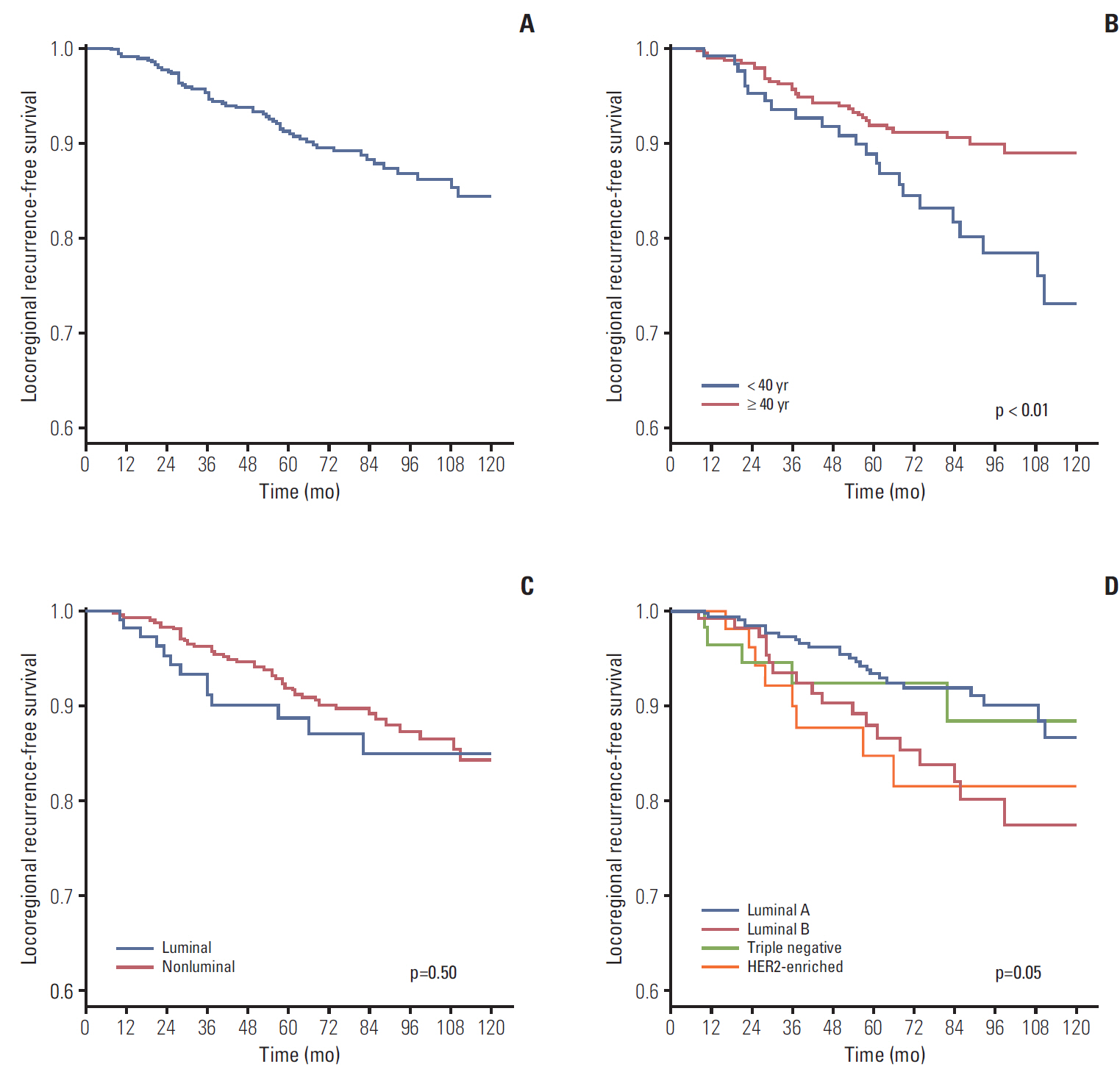
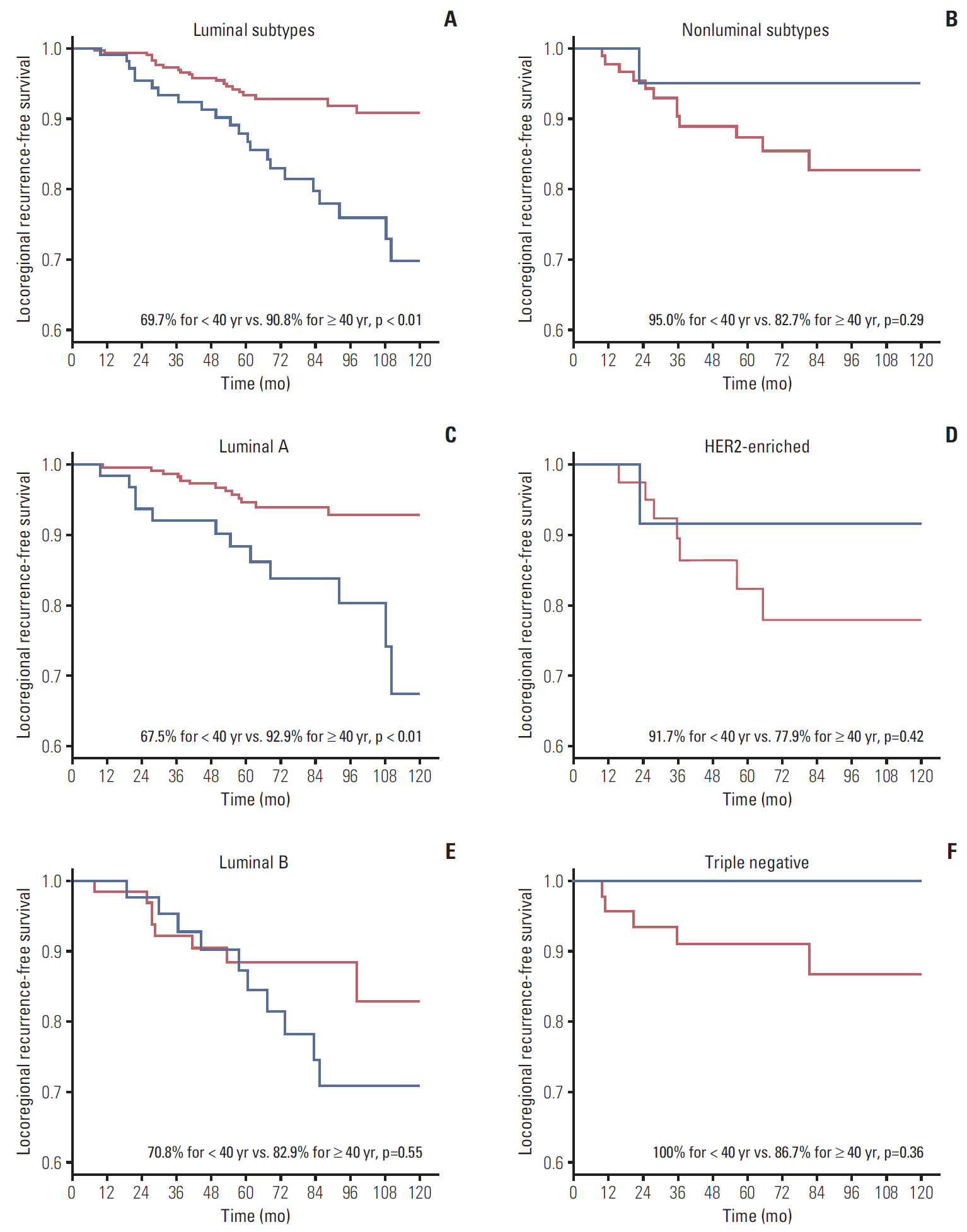
During median follow-up of 84 months, the 10-year locoregional recurrence-free survival rate (LRRFS) was 84.3% for all patients. Patients < 40 years showed significantly worse 10-year LRRFS than those ≥ 40 years (73.2% vs. 89.0%, respectively; p=0.01). The negative effect of young age on LRRFS was only observed in luminal subtypes (69.7% for < 40 years vs. 90.8% for ≥ 40 years; p < 0.01). Multivariate analysis using luminal subtypes ≥ 40 years as a reference revealed luminal subtypes < 40 years were significantly associated with increased risk of LRR (hazard ratio, 2.33; p < 0.01).
CONCLUSION
Young breast cancer patients with positive lymph nodes had a higher risk of LRR than those aged ≥ 40 years. This detrimental effect of young age on LRR was confined in luminal subtypes.
MeSH Terms
Figure
Cited by 1 articles
-
The different prognostic impact of age according to individual molecular subtypes in breast cancer
Nam Hee Kim, Hye Won Bang, Yong Hwa Eom, Seung Hye Choi
Ann Surg Treat Res. 2022;103(3):129-144. doi: 10.4174/astr.2022.103.3.129.
Reference
-
References
1. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–52.
Article2. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–74.
Article3. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–60.
Article4. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004; 10:5367–74.
Article5. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–502.
Article6. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006; 24:5652–7.
Article7. Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008; 26:1419–26.
Article8. Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008; 26:2373–8.
Article9. Millar EK, Graham PH, O'Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009; 27:4701–8.
Article10. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010; 28:1684–91.
Article11. Gabos Z, Thoms J, Ghosh S, Hanson J, Deschenes J, Sabri S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010; 124:187–94.
Article12. Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011; 29:3885–91.
Article13. Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013; 31:3083–90.
Article14. Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986; 315:559–63.
Article15. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008; 26:3324–30.
Article16. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–8.17. Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (< 35 years) with operable breast cancer. Ann Oncol. 2010; 21:1974–81.18. Kim HJ, Han W, Yi OV, Shin HC, Ahn SK, Koh BS, et al. Young age is associated with ipsilateral breast tumor recurrence after breast conserving surgery and radiation therapy in patients with HER2-positive/ER-negative subtype. Breast Cancer Res Treat. 2011; 130:499–505.
Article19. Kim EK, Noh WC, Han W, Noh DY. Prognostic significance of young age (< 35 years) by subtype based on ER, PR, and HER2 status in breast cancer: a nationwide registry-based study. World J Surg. 2011; 35:1244–53.20. Park YH, Lee SJ, Jung HA, Kim SM, Kim MJ, Kil WH, et al. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast. 2015; 24:213–7.
Article21. Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: are there age differentials? J Natl Cancer Inst Monogr. 1994; (16):35–42.22. Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Orlando L, Ghisini R, et al. Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol. 2006; 17:1497–503.
Article23. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013; 381:805–16.24. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–50.
Article25. Kim J, Han W, Jung SY, Park YH, Moon HG, Ahn SK, et al. The value of Ki67 in very young women with hormone receptor-positive breast cancer: retrospective analysis of 9,321 Korean women. Ann Surg Oncol. 2015; 22:3481–8.
Article26. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.27. Sheridan W, Scott T, Caroline S, Yvonne Z, Vanessa B, David V, et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat. 2014; 147:617–29.
Article28. Liedtke C, Hess KR, Karn T, Rody A, Kiesel L, Hortobagyi GN, et al. The prognostic impact of age in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2013; 138:591–9.
Article29. Han W, Kim SW, Park IA, Kang D, Youn YK, Oh SK, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004; 4:82.
Article30. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008; 14:1368–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognosis according to the timing of recurrence in breast cancer
- Subtype Is a Predictive Factor of Nonsentinel Lymph Node Involvement in Sentinel Node-Positive Breast Cancer Patients
- The different prognostic impact of age according to individual molecular subtypes in breast cancer
- Potential Benefits of Neoadjuvant Chemotherapy in Clinically Node-Positive Luminal Subtypeâ» Breast Cancer
- Predictive Value of Molecular Subtyping for Locoregional Recurrence in Early-Stage Breast Cancer with N1 without Postmastectomy Radiotherapy