Cancer Res Treat.
2017 Apr;49(2):473-483. 10.4143/crt.2016.166.
Long-Term Outcome of Distal Cholangiocarcinoma after Pancreaticoduodenectomy Followed by Adjuvant Chemoradiotherapy: A 15-Year Experience in a Single Institution
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea.
- 2Division of Biological Warfare Preparedness and Response, Armed Forces Medical Research Institute, Daejeon, Korea.
- 3Department of Radiation Oncology, Ewha Womans University School of Medicine, Seoul, Korea. kyubokim.ro@gmail.com
- 4Department of Radiation Oncology, Chungnam National University School of Medicine, Daejeon, Korea.
- 5Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2378120
- DOI: http://doi.org/10.4143/crt.2016.166
Abstract
- PURPOSE
This study was conducted to evaluate the long-term outcome in patients undergoing pancreaticoduodenectomy (PD) followed by adjuvant chemoradiotherapy for distal cholangiocarcinoma (DCC) in a high-volume center and to identify the prognostic impact of clinicopathologic factors.
MATERIALS AND METHODS
A total of 132 consecutive patients who met the inclusion criteria were retrieved from the institutional database from January 1995 to September 2009. All patients received adjuvant treatments at a median of 45 days after the surgery. Median follow-up duration was 57 months (range, 6 to 225 months) for all patients and 105 months for survivors (range, 13 to 225 months).
RESULTS
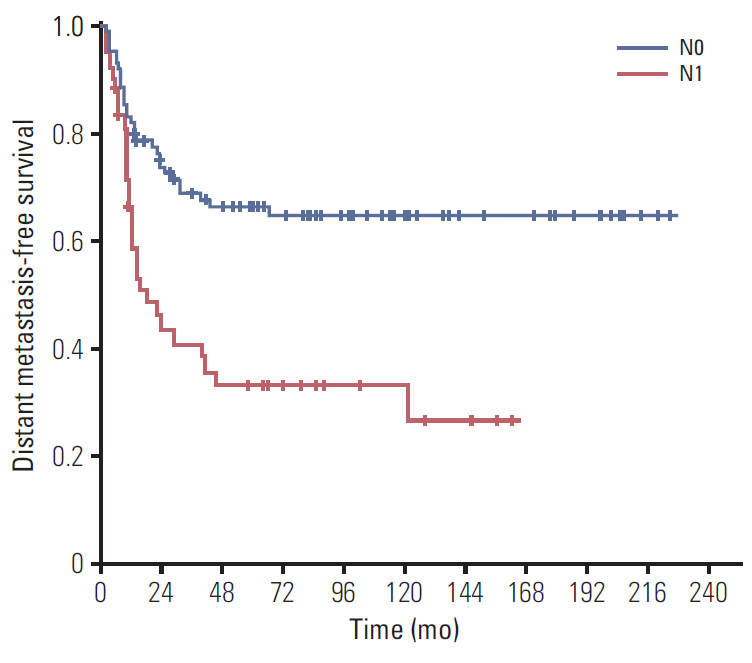
The 5-year locoregional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) rates were 70.7%, 55.7%, 49.4%, and 48.1%, respectively. Univariate analysis revealed poorly differentiated (P/D) tumors and lymph node (LN) metastasis were significantly associated with DMFS and OS. Additionally, preoperative carbohydrate antigen 19-9 level was significantly correlated with DFS, LRRFS, and DMFS. Upon multivariate analysis for OS, P/D tumors (p=0.015) and LN metastasis (p=0.003) were significant prognosticators that predicted inferior OS. Grade 3 or higher late gastrointestinal toxicity occurred in only one patient (0.8%).
CONCLUSION
Adjuvant chemoradiotherapy after PD for DCC is an effective and tolerable strategy without significant side effects. During long-term follow-up, we found that prognosis of DCC was mainly influenced by histologic differentiation and LN metastasis. For patients with these risk factors, further research should focus on improving adjuvant strategies as well as other treatment approaches.
MeSH Terms
Figure
Cited by 1 articles
-
The prognostic value of the lymph node ratio in patients with distal cholangiocarcinoma after curative intended surgery: A single-center retrospective study
Chaeyung Oh, Hee Joon Kim, Sang Hwa Song, Eun Kyu Park, Young Hoe Hur, Yang Seok Koh, Chol Kyoon Cho
Ann Hepatobiliary Pancreat Surg. 2022;26(2):168-177. doi: 10.14701/ahbps.21-126.
Reference
-
References
1. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article2. Andrianello S, Paiella S, Allegrini V, Ramera M, Pulvirenti A, Malleo G, et al. Pancreaticoduodenectomy for distal cholangiocarcinoma: surgical results, prognostic factors, and long-term follow-up. Langenbecks Arch Surg. 2015; 400:623–8.
Article3. Koo TR, Eom KY, Kim IA, Cho JY, Yoon YS, Hwang DW, et al. Patterns of failure and prognostic factors in resected extrahepatic bile duct cancer: implication for adjuvant radiotherapy. Radiat Oncol J. 2014; 32:63–9.
Article4. Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, et al. Prognostic factors for survival after curative resection of distal cholangiocarcinoma: perineural invasion and lymphovascular invasion. Surg Today. 2014; 44:1879–86.5. Tan X, Xiao K, Liu W, Chang S, Zhang T, Tang H. Prognostic factors of distal cholangiocarcinoma after curative surgery: a series of 84 cases. Hepatogastroenterology. 2013; 60:1892–5.6. van der Gaag NA, Kloek JJ, de Bakker JK, Musters B, Geskus RB, Busch OR, et al. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol. 2012; 23:2642–9.
Article7. Kamposioras K, Anthoney A, Fernandez Moro C, Cairns A, Smith AM, Liaskos C, et al. Impact of intrapancreatic or extrapancreatic bile duct involvement on survival following pancreatoduodenectomy for common bile duct cancer. Br J Surg. 2014; 101:89–99.
Article8. Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, et al. Distant metastasis risk stratification for patients undergoing curative resection followed by adjuvant chemoradiation for extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2012; 84:81–7.
Article9. Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009; 16:1–7.
Article10. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463–73.11. Kwon HJ, Kim SG, Chun JM, Lee WK, Hwang YJ. Prognostic factors in patients with middle and distal bile duct cancers. World J Gastroenterol. 2014; 20:6658–65.
Article12. Wiltberger G, Krenzien F, Benzing C, Atanasov G, Klein F, Hau HM, et al. Prognostic accuracy of the seventh edition of the TNM classification compared with the fifth and sixth edition for distal cholangiocarcinoma. Ann Surg Oncol. 2016; 23:1320–6.
Article13. Zhou Y, Liu S, Wu L, Wan T. Survival after surgical resection of distal cholangiocarcinoma: a systematic review and metaanalysis of prognostic factors. Asian J Surg. 2017; 40:129–38.
Article14. Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D'Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010; 251:675–81.
Article15. Sakamoto Y, Shimada K, Nara S, Esaki M, Ojima H, Sano T, et al. Surgical management of infrahilar/suprapancreatic cholangiocarcinoma: an analysis of the surgical procedures, surgical margins, and survivals of 77 patients. J Gastrointest Surg. 2010; 14:335–43.
Article16. Endo I, House MG, Klimstra DS, Gonen M, D'Angelica M, Dematteo RP, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008; 15:2104–12.
Article17. Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005; 103:1210–6.
Article18. Sasaki R, Takeda Y, Funato O, Nitta H, Kawamura H, Uesugi N, et al. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007; 31:1788–96.
Article19. Nakanishi Y, Kondo S, Zen Y, Yonemori A, Kubota K, Kawakami H, et al. Impact of residual in situ carcinoma on postoperative survival in 125 patients with extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Sci. 2010; 17:166–73.
Article20. Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, et al. Adjuvant chemoradiotherapy after curative resection for extrahepatic bile duct cancer: a long-term single center experience. Am J Clin Oncol. 2012; 35:136–40.21. Park JH, Choi EK, Ahn SD, Lee SW, Song SY, Yoon SM, et al. Postoperative chemoradiotherapy for extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2011; 79:696–704.
Article22. Kim JK, Ha HK, Han DJ, Auh YH. CT analysis of postoperative tumor recurrence patterns in periampullary cancer. Abdom Imaging. 2003; 28:384–91.
Article23. de Castro SM, Kuhlmann KF, van Heek NT, Busch OR, Offerhaus GJ, van Gulik TM, et al. Recurrent disease after microscopically radical (R0) resection of periampullary adenocarcinoma in patients without adjuvant therapy. J Gastrointest Surg. 2004; 8:775–84.24. Oyasiji T, Zhang J, Kuvshinoff B, Iyer R, Hochwald SN. Molecular targets in biliary carcinogenesis and implications for therapy. Oncologist. 2015; 20:742–51.
Article25. Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: current status and emerging strategies. World J Gastrointest Oncol. 2015; 7:338–46.
Article26. Kim YS, Hwang IG, Park SE, Go SI, Kang JH, Park I, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol. 2016; 77:979–85.
Article27. Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Moon SH, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011; 81:e853.
Article28. Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 2005; 241:77–84.
Article29. Han IW, Jang JY, Lee KB, Kang MJ, Kwon W, Park JW, et al. Clinicopathological analysis and prognosis of extrahepatic bile duct cancer with a microscopic positive ductal margin. HPB (Oxford). 2014; 16:575–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cholangiocarcinoma in choledochal cyst after cystoenterostomy: how a mistreated choledochal cyst can progress to malignancy
- Validation of the Effectiveness and Safety of Temozolomide during and after Radiotherapy for Newly Diagnosed Glioblastomas: 10-year Experience of a Single Institution
- Impact of Nrf2 overexpression on cholangiocarcinoma treatment and clinical prognosis
- Adjuvant Chemotherapy Versus Chemoradiation for Patients with Resected Pancreatic Adenocarcinoma: A Single-Center Retrospective Study
- One hundred sixty pancreaticoduodenectomies for periampullary cancers in a growing-volume setting: a single-institution and a single-surgeon's experience