J Korean Med Sci.
2017 Jun;32(6):908-918. 10.3346/jkms.2017.32.6.908.
Increased Circulating T Lymphocytes Expressing HLA-DR in Kidney Transplant Recipients with Microcirculation Inflammation
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. drcdkim@knu.ac.kr
- 2Department of Pathology, Kyungpook National University School of Medicine, Daegu, Korea.
- 3Department of Surgery, Kyungpook National University School of Medicine, Daegu, Korea.
- 4Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2377719
- DOI: http://doi.org/10.3346/jkms.2017.32.6.908
Abstract
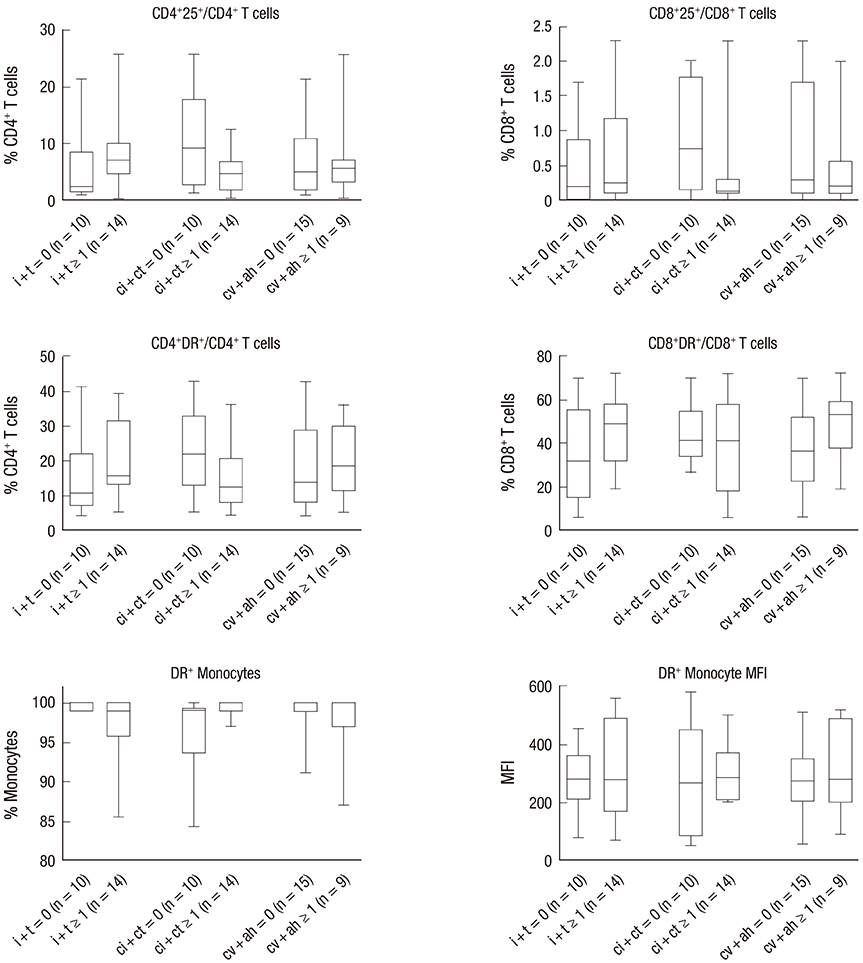
- We consecutively enrolled 82 kidney transplant recipients (KTRs) with stable renal function and 24 KTRs who underwent indication biopsy to compare the histological grading of renal allografts with the activity of circulating T lymphocyte subsets and monocytes determined by flow cytometry, which were obtained at 2 weeks after kidney transplantation (KT) and at the time of indication biopsy, respectively. The sum of the scores of glomerulitis (g) + peritubular capillaritis (ptc), inflammation (i) + tubulitis (t), interstitial fibrosis (ci) + tubular atrophy (ct), and fibrointimal thickening (cv) + arteriolar hyaline thickening (ah) was used to assign a histological grade to the renal allograft samples. The frequencies of CD4âºHLA-DRâº/CD4⺠T cells and CD8âºHLA-DRâº/CD8⺠T cells were significantly increased in KTRs with a microcirculation inflammation (MI) sum score ≥ 1 when compared with KTRs with an MI sum score = 0 as well as stable KTRs. In these 2 subsets, only CD4âºHLA-DRâº/CD4⺠T cells were positively correlated with MI sum scores. Analysis using the receiver operating characteristic (ROC) curve showed that antibody-mediated rejection (AMR) could be predicted with a sensitivity of 80.0% and a specificity of 94.7%, using a cutoff value of 29.6% frequency of CD4âºHLA-DRâº/CD4⺠T cells. MI was significantly associated with an increased frequency of activated T lymphocytes expressing human leukocyte antigen-antigen D related (HLA-DR). Further studies should focus on validating the utility of circulating CD4âºHLA-DRâº/CD4⺠T cells as a noninvasive, immunologic monitoring tool for the prediction of AMR.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Characteristics and Clinical Significance of De Novo Donor-Specific Anti-HLA Antibodies after Kidney Transplantation
Hee-Yeon Jung, Su-Hee Kim, Min-Young Seo, Sun-Young Cho, Youngae Yang, Ji-Young Choi, Jang-Hee Cho, Sun-Hee Park, Yong-Lim Kim, Hyung-Kee Kim, Seung Huh, Dong Il Won, Chan-Duck Kim
J Korean Med Sci. 2018;33(34):. doi: 10.3346/jkms.2018.33.e217.
Reference
-
1. Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009; 9:2520–2531.2. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–283.3. de Kort H, Willicombe M, Brookes P, Dominy KM, Santos-Nunez E, Galliford JW, Chan K, Taube D, McLean AG, Cook HT, et al. Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant. 2013; 13:485–492.4. Tufveson G, Forsum U, Claesson K, Klareskog L, Larsson E, Karlsson-Parra A, Frödin L. T-lymphocyte subsets and HLA-DR-expressing cells in rejected human kidney grafts. Scand J Immunol. 1983; 18:37–40.5. Bishop GA, Hall BM, Duggin GG, Horvath JS, Sheil AG, Tiller DJ. Immunopathology of renal allograft rejection analyzed with monoclonal antibodies to mononuclear cell markers. Kidney Int. 1986; 29:708–717.6. Girlanda R, Kleiner DE, Duan Z, Ford EA, Wright EC, Mannon RB, Kirk AD. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008; 8:600–607.7. Le Meur Y, Jose MD, Mu W, Atkins RC, Chadban SJ. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation. 2002; 73:1318–1324.8. Beik AI, Bateman WJ, Morris AG, Higgins RM, Lam FT. Monitoring of T-lymphocyte subsets in acute renal allograft rejection. Transplant Proc. 1998; 30:168–171.9. Kim SH, Oh EJ, Ghee JY, Song HK, Han DH, Yoon HE, Choi BS, Yoon SK, Choi JY, Moon IS, et al. Clinical significance of monitoring circulating CD4+CD25+ regulatory T cells in kidney transplantation during the early posttransplant period. J Korean Med Sci. 2009; 24:Suppl. S135–S142.10. Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010; 15:42–48.11. Hayde N, Broin PO, Bao Y, de Boccardo G, Lubetzky M, Ajaimy M, Pullman J, Colovai A, Golden A, Akalin E. Increased intragraft rejection-associated gene transcripts in patients with donor-specific antibodies and normal biopsies. Kidney Int. 2014; 86:600–609.12. Mueller TF, Einecke G, Reeve J, Sis B, Mengel M, Jhangri GS, Bunnag S, Cruz J, Wishart D, Meng C, et al. Microarray analysis of rejection in human kidney transplants using pathogenesis-based transcript sets. Am J Transplant. 2007; 7:2712–2722.13. Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009; 9:2312–2323.14. Hidalgo LG, Sis B, Sellares J, Campbell PM, Mengel M, Einecke G, Chang J, Halloran PF. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010; 10:1812–1822.15. Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013; 13:971–983.16. Halloran PF, Pereira AB, Chang J, Matas A, Picton M, De Freitas D, Bromberg J, Serón D, Sellarés J, Einecke G, et al. Microarray diagnosis of antibody-mediated rejection in kidney transplant biopsies: an international prospective study (INTERCOM). Am J Transplant. 2013; 13:2865–2874.17. Aquino-Dias EC, Joelsons G, da Silva DM, Berdichevski RH, Ribeiro AR, Veronese FJ, Gonçalves LF, Manfro RC. Non-invasive diagnosis of acute rejection in kidney transplants with delayed graft function. Kidney Int. 2008; 73:877–884.18. Graziotto R, Del Prete D, Rigotti P, Anglani F, Baldan N, Furian L, Valente M, Antonello A, Marchini F, D'Angelo A, et al. Perforin, granzyme B, and fas ligand for molecular diagnosis of acute renal-allograft rejection: analyses on serial biopsies suggest methodological issues. Transplantation. 2006; 81:1125–1132.19. Veale JL, Liang LW, Zhang Q, Gjertson DW, Du Z, Bloomquist EW, Jia J, Qian L, Wilkinson AH, Danovitch GM, et al. Noninvasive diagnosis of cellular and antibody-mediated rejection by perforin and granzyme B in renal allografts. Hum Immunol. 2006; 67:777–786.20. Simon T, Opelz G, Wiesel M, Ott RC, Süsal C. Serial peripheral blood perforin and granzyme B gene expression measurements for prediction of acute rejection in kidney graft recipients. Am J Transplant. 2003; 3:1121–1127.21. Sabek O, Dorak MT, Kotb M, Gaber AO, Gaber L. Quantitative detection of T-cell activation markers by real-time PCR in renal transplant rejection and correlation with histopathologic evaluation. Transplantation. 2002; 74:701–707.22. Townamchai N, Safa K, Chandraker A. Immunologic monitoring in kidney transplant recipients. Kidney Res Clin Pract. 2013; 32:52–61.23. Heng B, Li Y, Shi L, Du X, Lai C, Cheng L, Su Z. A meta-analysis of the significance of granzyme b and perforin in noninvasive diagnosis of acute rejection after kidney transplantation. Transplantation. 2015; 99:1477–1486.24. Näther BJ, Nickel P, Bold G, Presber F, Schönemann C, Pratschke J, Volk HD, Reinke P. Modified ELISPOT technique--highly significant inverse correlation of post-Tx donor-reactive IFNgamma-producing cell frequencies with 6 and 12 months graft function in kidney transplant recipients. Transpl Immunol. 2006; 16:232–237.25. Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005; 5:1971–1975.26. Nickel P, Presber F, Bold G, Biti D, Schönemann C, Tullius SG, Volk HD, Reinke P. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation. 2004; 78:1640–1646.27. Sengul S, Keven K, Gormez U, Kutlay S, Erturk S, Erbay B. Identification of patients at risk of acute rejection by pretransplantation and posttransplantation monitoring of soluble CD30 levels in kidney transplantation. Transplantation. 2006; 81:1216–1219.28. Breimer ME, Rydberg L, Jackson AM, Lucas DP, Zachary AA, Melancon JK, Von Visger J, Pelletier R, Saidman SL, Williams WW Jr, et al. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009; 87:549–556.29. Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012; 12:1168–1179.30. Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009; 9:2561–2570.31. Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012; 8:670–678.32. Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012; 217:216–224.33. Dunkelberger JR, Song WC. Role and mechanism of action of complement in regulating T cell immunity. Mol Immunol. 2010; 47:2176–2186.34. Haveman JW, van den Berg AP, van den Berk JM, Mesander G, Slooff MJ, de Leij LH, The TH. Low HLA-DR expression on peripheral blood monocytes predicts bacterial sepsis after liver transplantation: relation with prednisolone intake. Transpl Infect Dis. 1999; 1:146–152.35. Hoffman JA, Weinberg KI, Azen CG, Horn MV, Dukes L, Starnes VA, Woo MS. Human leukocyte antigen-DR expression on peripheral blood monocytes and the risk of pneumonia in pediatric lung transplant recipients. Transpl Infect Dis. 2004; 6:147–155.36. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969.37. Rowshani AT, Vereyken EJ. The role of macrophage lineage cells in kidney graft rejection and survival. Transplantation. 2012; 94:309–318.38. Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005; 68:1866–1874.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Donor Factors Influencing the Graft Survival of Kidney Transplants
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome
- Effect of suction blister fluid and IFN-gamma on HLA-A,B,C and HLA-DR espression in cultures human melanocytes
- Association of human leukocyte antigen homozygosity and de novo malignancies in kidney transplant recipients
- Gamma interferon modulates epidermal cell proliferation and mixed epidermal cell-lymphocyte reaction