Ann Surg Treat Res.
2017 Mar;92(3):117-122. 10.4174/astr.2017.92.3.117.
Significance of micrometastases in the calculation of the lymph node ratio for papillary thyroid cancer
- Affiliations
-
- 1Department of Breast Endocrine Surgery, Korea University College of Medicine, Seoul, Korea. gsson@korea.ac.kr
- KMID: 2377552
- DOI: http://doi.org/10.4174/astr.2017.92.3.117
Abstract
- PURPOSE
The lymph node ratio (LNR) is an important prognostic factor in papillary thyroid carcinoma (PTC), but micrometastases in cervical lymph nodes (LNs) are not of great clinical importance. In this study, we analyzed the accuracy of prediction of the prognosis depending on whether micrometastases were included in the number of metastatic LNs when calculating LNR.
METHODS
The study included 353 PTC patients who underwent total thyroidectomy with neck LN dissection, and calculated LNR by 2 methods according to whether micrometastases were included in the number of metastatic LNs: Method 1 did not and method 2 did include. To compare the predictive values of LNR by the 2 methods, correlation coefficients and receiver operating characteristic (ROC) curves were analyzed.
RESULTS
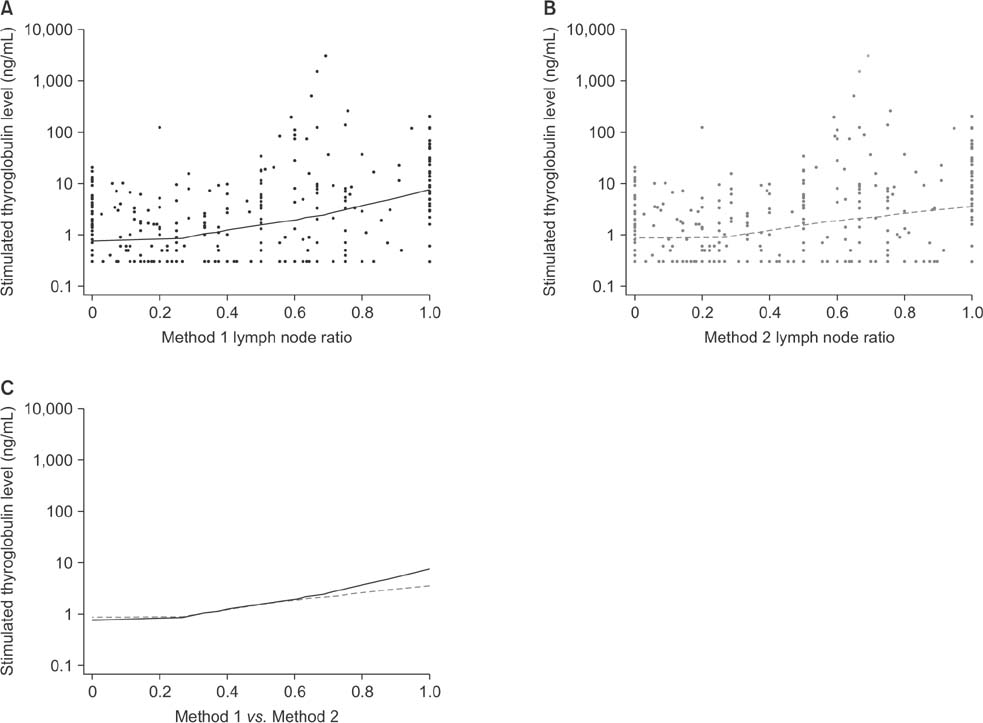
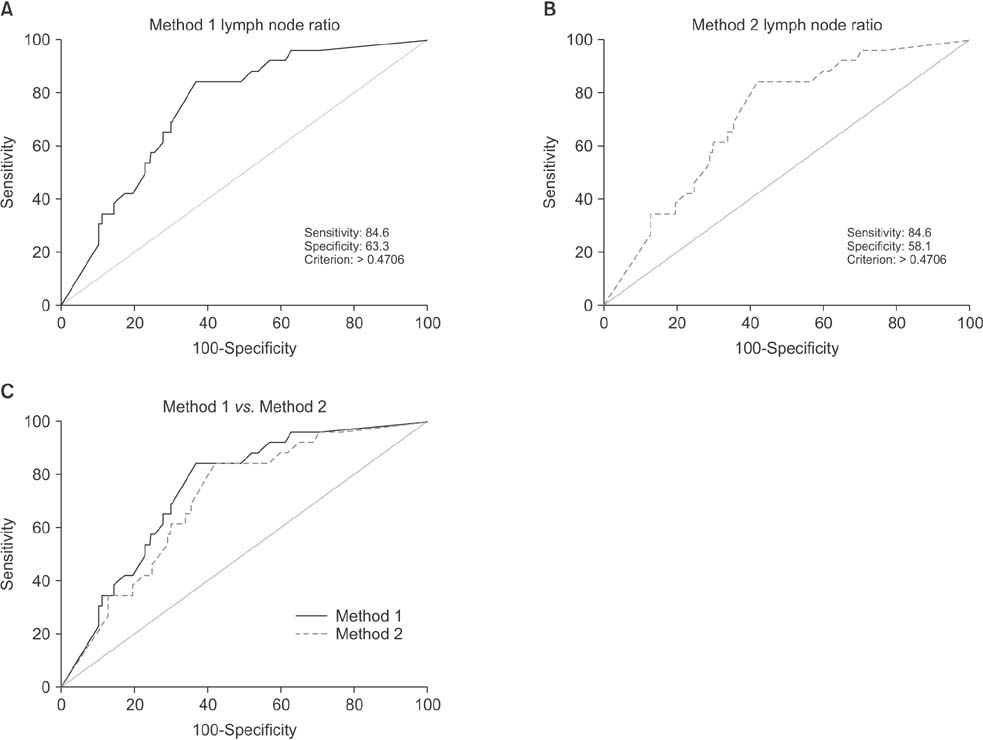
Positive correlations were found between LNR and preablation stimulated thyroglobulin (sTg) levels in both methods, but the correlation between method 1 LNR and preablation sTg level was significantly stronger than that for method 2 (Fisher z = 1.7, P = 0.045). The areas under these 2 independent ROC curves were analyzed; the prognostic efficacy of method 1 LNR was more accurate than that of method 2 LNR, and the difference was statistically significant (P = 0.0001).
CONCLUSION
Regional recurrence of PTC can be predicted more accurately by not including micrometastases in the number of metastatic LNs when calculating LNR.
Keyword
MeSH Terms
Figure
Reference
-
1. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71:414–424.2. Tubiana M, Schlumberger M, Rougier P, Laplanche A, Benhamou E, Gardet P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985; 55:794–804.3. Samaan NA, Maheshwari YK, Nader S, Hill CS Jr, Schultz PN, Haynie TP, et al. Impact of therapy for differentiated carcinoma of the thyroid: an analysis of 706 cases. J Clin Endocrinol Metab. 1983; 56:1131–1138.4. Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg. 2001; 88:1241–1244.5. Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988; 104:954–962.6. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.7. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007; 245:604–610.8. Chang YW, Kim HS, Kim HY, Lee JB, Bae JW, Son GS. Should central lymph node dissection be considered for all papillary thyroid microcarcinoma? Asian J Surg. 2016; 39:197–201.9. Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014; 21:277–283.10. Lang BH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012; 19:1257–1263.11. Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol. 2013; 168:219–225.12. Ito Y, Fukushima M, Kihara M, Takamura Y, Kobayashi K, Miya A, et al. Investigation of the prognosis of patients with papillary thyroid carcinoma by tumor size. Endocr J. 2012; 59:457–464.13. Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ, Lee BJ. Clinical implication of the number of central lymph node metastasis in papillary thyroid carcinoma: preliminary report. World J Surg. 2010; 34:2558–2563.14. Cranshaw IM, Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol. 2008; 17:253–258.15. Teixeira G, Teixeira T, Gubert F, Chikota H, Tufano R. The incidence of central neck micrometastatic disease in patients with papillary thyroid cancer staged preoperatively and intraoperatively as N0. Surgery. 2011; 150:1161–1167.16. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002; 131:249–256.17. Huvos AG, Hutter RV, Berg JW. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971; 173:44–46.18. Beal SH, Chen SL, Schneider PD, Martinez SR. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010; 76:28–32.19. Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2013; 20:1906–1911.20. Yip J, Orlov S, Orlov D, Vaisman A, Hernandez KG, Etarsky D, et al. Predictive value of metastatic cervical lymph node ratio in papillary thyroid carcinoma recurrence. Head Neck. 2013; 35:592–598.21. Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013; 18:157–162.22. Lee YS, Kim SW, Kim SW, Kim SK, Kang HS, Lee ES, et al. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg. 2007; 31:1954–1959.23. Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013; 14:297–305.24. Pacini F, Lari R, Mazzeo S, Grasso L, Taddei D, Pinchera A. Diagnostic value of a single serum thyroglobulin determination on and off thyroid suppressive therapy in the follow-up of patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 1985; 23:405–411.25. Chang YW, Kim HS, Jung SP, Kim HY, Lee JB, Bae JW, et al. Pre-ablation stimulated thyroglobulin is a better predictor of recurrence in pathological N1a papillary thyroid carcinoma than the lymph node ratio. Int J Clin Oncol. 2016; 21:862–868.26. Lee SG, Ho J, Choi JB, Kim TH, Kim MJ, Ban EJ, et al. Optimal cut-off values of lymph node ratio predicting recurrence in papillary thyroid cancer. Medicine (Baltimore). 2016; 95:e2692.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- One Case of a Primary Papillary Thyroid Carcinoma in the Intrathoracic Lymph Node
- A Case of Cystic Lymph Node Metastasis from Thyroid Papillary Microcarcinoma
- Co-existence of Papillary Thyroid Cancer with Tuberculosis Involving the Thyroid and Ipsilateral Paratracheal Lymph Node: A Case Report
- The Pattern of Cervical Lymph Node Metastases in Papillary Thyroid Cancer
- Retropharyngeal Lymph Node Metastasis of Thyroid Papillary Carcinoma