Korean Circ J.
2017 Mar;47(2):270-277. 10.4070/kcj.2016.0213.
Diverse Phenotypic Expression of Cardiomyopathies in a Family with TNNI3 p.Arg145Trp Mutation
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dukkyung.kim@gmail.com
- 2Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2377469
- DOI: http://doi.org/10.4070/kcj.2016.0213
Abstract
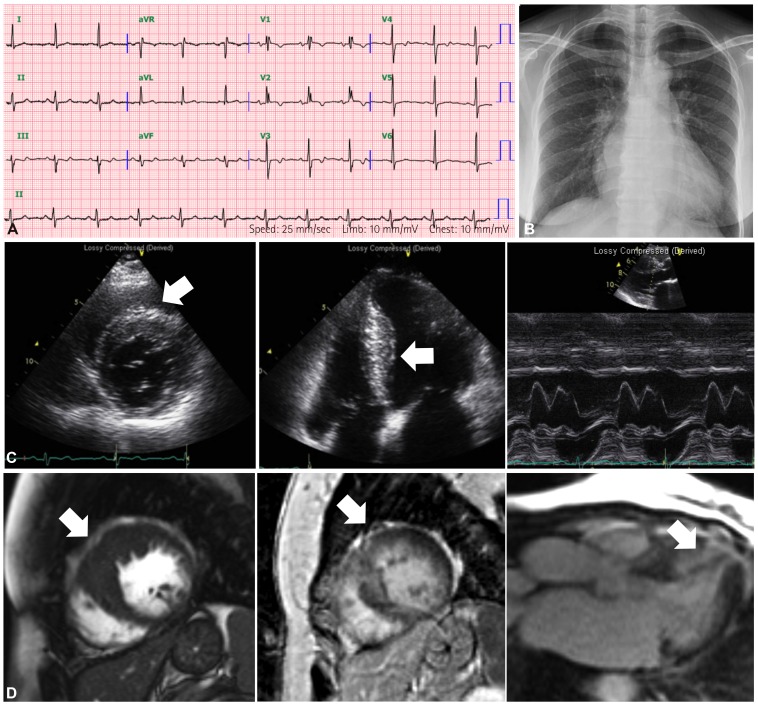
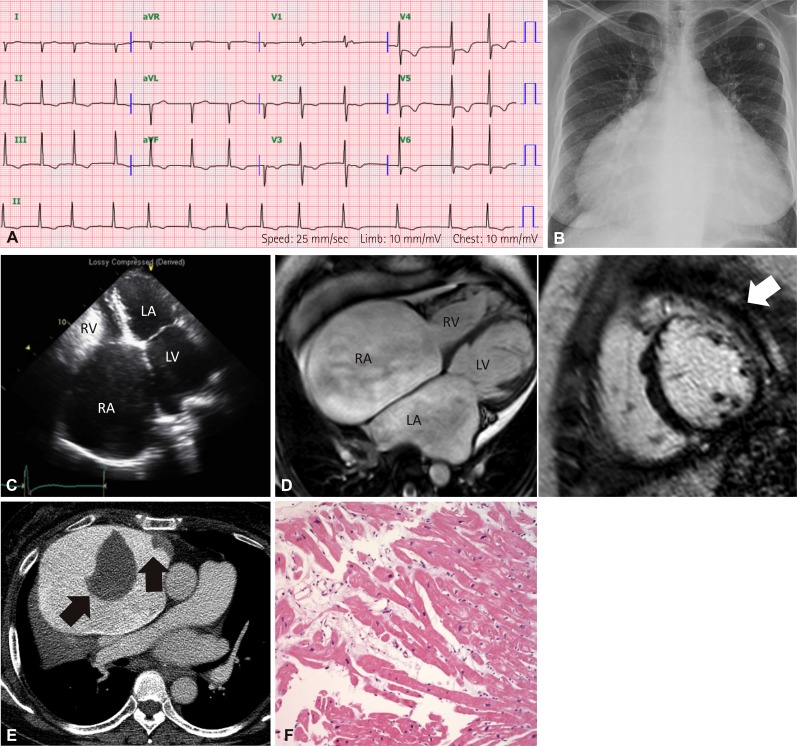
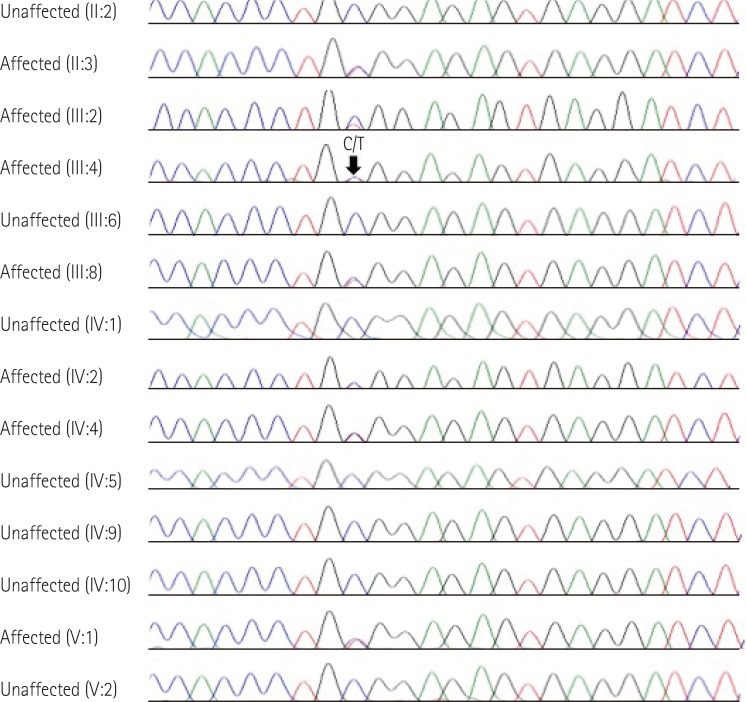
- Genetic diagnosis of cardiomyopathies is challenging, due to the marked genetic and allelic heterogeneity and the lack of knowledge of the mutations that lead to clinical phenotypes. Here, we present the case of a large family, in which a single TNNI3 mutation caused variable phenotypic expression, ranging from restrictive cardiomyopathy (RCMP) to hypertrophic cardiomyopathy (HCMP) to near-normal phenotype. The proband was a 57-year-old female with HCMP. Examining the family history revealed that her elder sister had expired due to severe RCMP. Using a next-generation sequencing-based gene panel to analyze the proband, we identified a known TNNI3 gene mutation, c.433C>T, which is predicted to cause an amino acid substitution (p.Arg145Trp) in the highly conserved inhibitory region of the cardiac troponin I protein. Sanger sequencing confirmed that six relatives with RCMP or near-normal phenotypes also carried this mutation. To our knowledge, this is the first genetically confirmed family with diverse phenotypic expression of cardiomyopathies in Korea. Our findings demonstrate familial implications, where a single mutation in a sarcomere protein can cause diverse phenotypic expression of cardiomyopathies.
Keyword
MeSH Terms
Figure
Reference
-
1. Tariq M, Ware SM. Importance of genetic evaluation and testing in pediatric cardiomyopathy. World J Cardiol. 2014; 6:1156–1165. PMID: 25429328.2. Callis TE, Jensen BC, Weck KE, Willis MS. Evolving molecular diagnostics for familial cardiomyopathies: at the heart of it all. Expert Rev Mol Diagn. 2010; 10:329–351. PMID: 20370590.3. Roma-Rodrigues C, Fernandes AR. Genetics of hypertrophic cardiomyopathy: advances and pitfalls in molecular diagnosis and therapy. Appl Clin Genet. 2014; 7:195–208. PMID: 25328416.4. Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol. 2010; 48:882–892. PMID: 19914256.5. Luedde M, Ehlermann P, Weichenhan D, et al. Severe familial left ventricular non-compaction cardiomyopathy due to a novel troponin T (TNNT2) mutation. Cardiovasc Res. 2010; 86:452–460. PMID: 20083571.6. Mogensen J, Kubo T, Duque M, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest. 2003; 111:209–216. PMID: 12531876.7. Vallins WJ, Brand NJ, Dabhade N, Butler-Browne G, Yacoub MH, Barton PJ. Molecular cloning of human cardiac troponin I using polymerase chain reaction. FEBS Lett. 1990; 270:57–61. PMID: 2226790.8. Bhavsar PK, Brand NJ, Yacoub MH, Barton PJ. Isolation and characterization of the human cardiac troponin I gene (TNNI3). Genomics. 1996; 35:11–23. PMID: 8661099.9. Gomes AV, Liang J, Potter JD. Mutations in human cardiac troponin I that are associated with restrictive cardiomyopathy affect basal ATPase activity and the calcium sensitivity of force development. J Biol Chem. 2005; 280:30909–30915. PMID: 15961398.10. Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006; 281:13471–13477. PMID: 16531415.11. Davis J, Wen H, Edwards T, Metzger JM. Allele and species dependent contractile defects by restrictive and hypertrophic cardiomyopathy-linked troponin I mutants. J Mol Cell Cardiol. 2008; 44:891–904. PMID: 18423659.12. Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem. 1999; 190:9–32. PMID: 10098965.13. Lang R, Gomes AV, Zhao J, Housmans PR, Miller T, Potter JD. Functional analysis of a troponin I (R145G) mutation associated with familial hypertrophic cardiomyopathy. J Biol Chem. 2002; 277:11670–11678. PMID: 11801593.14. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000; 342:1077–1084. PMID: 10760308.15. Webber SA, Lipshultz SE, Sleeper LA, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation. 2012; 126:1237–1244. PMID: 22843787.16. Zou Y, Wang J, Liu X, et al. Multiple gene mutations, not the type of mutation, are the modifier of left ventricle hypertrophy in patients with hypertrophic cardiomyopathy. Mol Biol Rep. 2013; 40:3969–3976. PMID: 23283745.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiomyopathies with Mixed and Inapparent Morphological Features in Cardiac Troponin I3 Mutation
- Phenotypic Difference of CLCN1 Gene Variant (A313T) in a Korean Family with Myotonia Congenita
- Epidermal Growth Factor Receptor Expression of Non-small Cell Carcinoma and Its Relationship with Genomic Mutation
- Two cases of 17α-hydroxylase/17,20-lyase deficiency caused by the CYP17A1 mutation
- A familial case with brachydactyly type C with a GDF5 mutation