Korean J Physiol Pharmacol.
2017 May;21(3):353-360. 10.4196/kjpp.2017.21.3.353.
Expression profile of mitochondrial voltage-dependent anion channel-1 (VDAC1) influenced genes is associated with pulmonary hypertension
- Affiliations
-
- 1Department of Physiology and Cell Biology, University of Nevada School of Medicine, Reno, NV 89557, USA.
- 2Department of Medicine, University of Arizona, Tucson, AZ 85721, USA.
- 3Department of Physiology, Nanjing Medical University, Nanjing, Jiangsu 211166, China.
- 4Section of Pulmonary, Critical Care, Sleep & Allergy, Department of Medicine, The University of Illinois at Chicago, Chicago, IL 60612, USA.
- 5Department of Physiology, College of Medicine, Chung-Ang University, Seoul 06974, Korea. akdongyi01@cau.ac.kr haena@cau.ac.kr
- KMID: 2376964
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.353
Abstract
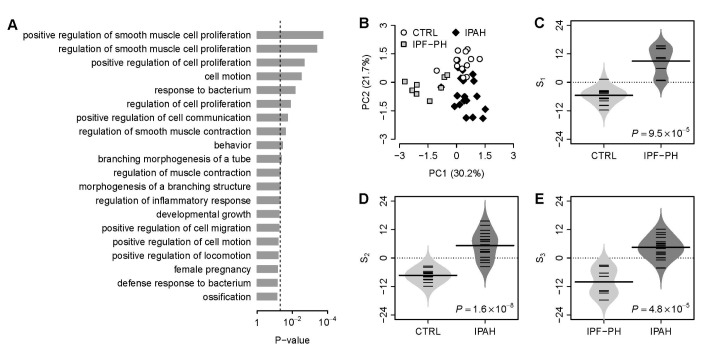
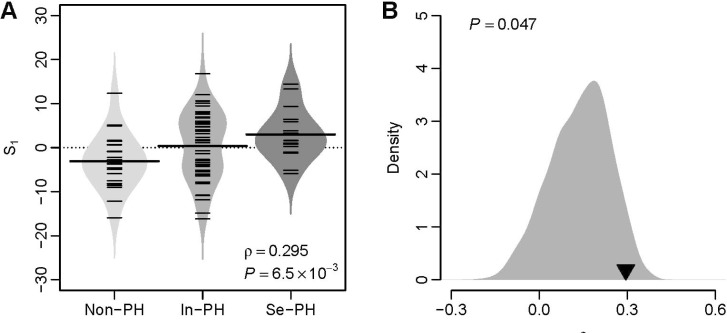
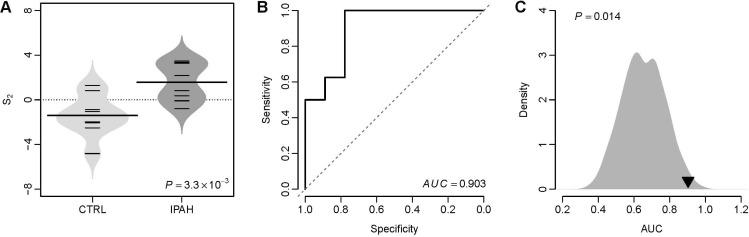
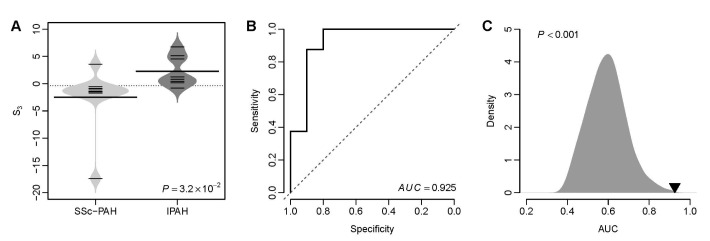
- Several human diseases have been associated with mitochondrial voltage-dependent anion channel-1 (VDAC1) due to its role in calcium ion transportation and apoptosis. Recent studies suggest that VDAC1 may interact with endothelium-dependent nitric oxide synthase (eNOS). Decreased VDAC1 expression may limit the physical interaction between VDAC1 and eNOS and thus impair nitric oxide production, leading to cardiovascular diseases, including pulmonary arterial hypertension (PAH). In this report, we conducted meta-analysis of genome-wide expression data to identify VDAC1 influenced genes implicated in PAH pathobiology. First, we identified the genes differentially expressed between wild-type and Vdac1 knockout mouse embryonic fibroblasts in hypoxic conditions. These genes were deemed to be influenced by VDAC1 deficiency. Gene ontology analysis indicates that the VDAC1 influenced genes are significantly associated with PAH pathobiology. Second, a molecular signature derived from the VDAC1 influenced genes was developed. We suggest that, VDAC1 has a protective role in PAH and the gene expression signature of VDAC1 influenced genes can be used to i) predict severity of pulmonary hypertension secondary to pulmonary diseases, ii) differentiate idiopathic pulmonary artery hypertension (IPAH) patients from controls, and iii) differentiate IPAH from connective tissue disease associated PAH.
MeSH Terms
-
Animals
Anoxia
Apoptosis
Calcium
Cardiovascular Diseases
Connective Tissue Diseases
Fibroblasts
Gene Expression
Gene Ontology
Humans
Hypertension
Hypertension, Pulmonary*
Ion Transport
Lung Diseases
Mice
Mice, Knockout
Nitric Oxide
Nitric Oxide Synthase
Pulmonary Artery
Transcriptome
Calcium
Nitric Oxide
Nitric Oxide Synthase
Figure
Reference
-
1. Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem. 2004; 256-257:107–115. PMID: 14977174.
Article2. Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007; 1768:2510–2515. PMID: 17617374.
Article3. Verrier F, Mignotte B, Jan G, Brenner C. Study of PTPC composition during apoptosis for identification of viral protein target. Ann N Y Acad Sci. 2003; 1010:126–142. PMID: 15033708.
Article4. Li L, Yao YC, Gu XQ, Che D, Ma CQ, Dai ZY, Li C, Zhou T, Cai WB, Yang ZH, Yang X, Gao GQ. Plasminogen kringle 5 induces endothelial cell apoptosis by triggering a voltage-dependent anion channel 1 (VDAC1) positive feedback loop. J Biol Chem. 2014; 289:32628–32638. PMID: 25296756.
Article5. Chu Y, Goldman JG, Kelly L, He Y, Waliczek T, Kordower JH. Abnormal alpha-synuclein reduces nigral voltage-dependent anion channel 1 in sporadic and experimental Parkinson's disease. Neurobiol Dis. 2014; 69:1–14. PMID: 24825319.
Article6. Huang H, Shah K, Bradbury NA, Li C, White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014; 5:e1482. PMID: 25341036.7. Ko JH, Gu W, Lim I, Zhou T, Bang H. Expression profiling of mitochondrial voltage-dependent anion channel-1 associated genes predicts recurrence-free survival in human carcinomas. PLoS One. 2014; 9:e110094. PMID: 25333947.
Article8. Alvira CM, Umesh A, Husted C, Ying L, Hou Y, Lyu SC, Nowak J, Cornfield DN. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am J Respir Cell Mol Biol. 2012; 47:669–678. PMID: 22842492.
Article9. Fish JE, Marsden PA. Endothelial nitric oxide synthase: insight into cell-specific gene regulation in the vascular endothelium. Cell Mol Life Sci. 2006; 63:144–162. PMID: 16416260.
Article10. Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006; 113:1708–1714. PMID: 16585403.11. Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest. 2002; 122(6 Suppl):326S–334S. PMID: 12475810.12. Tang H, Ayon RJ, Yuan JX. New insights into the pathology of pulmonary hypertension: implication of the miR-210/ISCU1/2/Fe-S axis. EMBO Mol Med. 2015; 7:689–691. PMID: 25851536.13. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002; 30:207–210. PMID: 11752295.
Article14. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001; 98:5116–5121. PMID: 11309499.
Article15. R_Development_Core_Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing;2005.16. Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002; 99:6567–6572. PMID: 12011421.
Article17. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. The Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet. 2000; 25:25–29. PMID: 10802651.
Article18. Ko JH, Ko EA, Gu W, Lim I, Bang H, Zhou T. Expression profiling of ion channel genes predicts clinical outcome in breast cancer. Mol Cancer. 2013; 12:106. PMID: 24053408.
Article19. Wang R, Gurguis CI, Gu W, Ko EA, Lim I, Bang H, Zhou T, Ko JH. Ion channel gene expression predicts survival in glioma patients. Sci Rep. 2015; 5:11593. PMID: 26235283.
Article20. Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010; 298:H1235–H1248. PMID: 20081107.
Article21. Mura M, Anraku M, Yun Z, McRae K, Liu M, Waddell TK, Singer LG, Granton JT, Keshavjee S, de Perrot M. Gene expression profiling in the lungs of patients with pulmonary hypertension associated with pulmonary fibrosis. Chest. 2012; 141:661–673. PMID: 21835902.
Article22. Venet D, Dumont JE, Detours V. Most random gene expression signatures are significantly associated with breast cancer outcome. PLoS Comput Biol. 2011; 7:e1002240. PMID: 22028643.
Article23. Ghosh MC, Zhang DL, Jeong SY, Kovtunovych G, Ollivierre-Wilson H, Noguchi A, Tu T, Senecal T, Robinson G, Crooks DR, Tong WH, Ramaswamy K, Singh A, Graham BB, Tuder RM, Yu ZX, Eckhaus M, Lee J, Springer DA, Rouault TA. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α. Cell Metab. 2013; 17:271–281. PMID: 23395173.
Article24. Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004; 286:C416–C425. PMID: 14561589.
Article25. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014; 9:157–179. PMID: 24050627.
Article26. Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007; 132:998–1006. PMID: 17873194.
Article27. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006; 98:1528–1537. PMID: 16709899.28. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999; 103:691–696. PMID: 10074486.29. Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003; 111:1519–1527. PMID: 12750401.30. Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res. 2005; 6:109. PMID: 16171515.
Article31. Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995; 95:1798–1807. PMID: 7706486.
Article32. Nadeau S, Baribeau J, Janvier A, Perreault T. Changes in expression of vascular endothelial growth factor and its receptors in neonatal hypoxia-induced pulmonary hypertension. Pediatr Res. 2005; 58:199–205. PMID: 16006432.
Article33. Ataga KI, Brittain JE, Jones SK, May R, Delaney J, Strayhorn D, Desai P, Redding-Lallinger R, Key NS, Orringer EP. Association of soluble fms-like tyrosine kinase-1 with pulmonary hypertension and haemolysis in sickle cell disease. Br J Haematol. 2011; 152:485–491. PMID: 21223248.
Article34. Perkett EA, Badesch DB, Roessler MK, Stenmark KR, Meyrick B. Insulin-like growth factor I and pulmonary hypertension induced by continuous air embolization in sheep. Am J Respir Cell Mol Biol. 1992; 6:82–87. PMID: 1728299.
Article35. Sun M, Ramchandran R, Chen J, Yang Q, Raj JU. Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice. Am J Respir Cell Mol Biol. 2016; 55:779–791. PMID: 27438786.
Article36. Yang Q, Sun M, Ramchandran R, Raj JU. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: Role of epigenetic regulation. Vascul Pharmacol. 2015; 73:20–31. PMID: 25921925.
Article37. Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008; 117:2114–2122. PMID: 18391113.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Annexin A5 on Mitochondria-Dependent Apoptosis Induced by Tetramethoxystilbene in Human Breast Cancer Cells
- Change of voltage-gated potassium channel 1.7 expressions in monocrotaline-induced pulmonary arterial hypertension rat model
- Protective Effect of Right Ventricular Mitochondrial Damage by Cyclosporine A in Monocrotaline-induced Pulmonary Hypertension
- Age-dependent expression of ion channel genes in rat
- The expression of voltage-dependent K+ channels in stria vascularis of the guinea pig cochlea