Korean J Physiol Pharmacol.
2017 May;21(3):309-316. 10.4196/kjpp.2017.21.3.309.
Activation of transient receptor potential vanilloid 3 by the methanolic extract of Schisandra chinensis fruit and its chemical constituent γ-schisandrin
- Affiliations
-
- 1Department of Physiology, Dongguk University College of Medicine, Gyeongju 38066, Korea. jh_nam@dongguk.ac.kr
- 2Channelopathy Research Center (CRC), Dongguk University College of Medicine, Goyang 10326, Korea. wk2kim@naver.com
- 3College of Pharmacy and Integrated Research Institute for Drug Development, Dongguk University-Seoul, Goyang 10326, Korea.
- 4Department of Sasang Constitutional Medicine, College of Korean Medicine, Dongguk University, Goyang 10326, Korea.
- 5Department of Internal Medicine, Graduate School of Medicine, Dongguk University, Goyang 10326, Korea.
- KMID: 2376959
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.309
Abstract
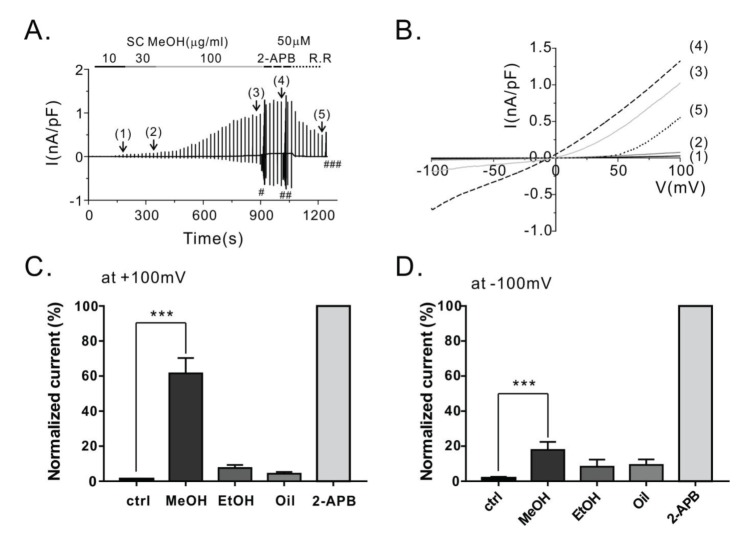
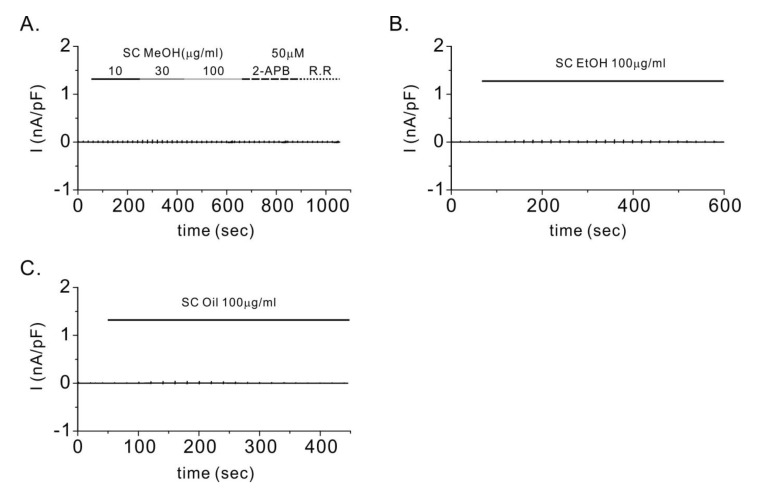
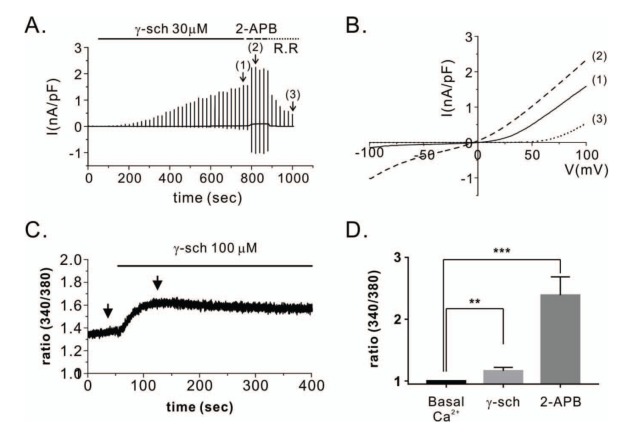
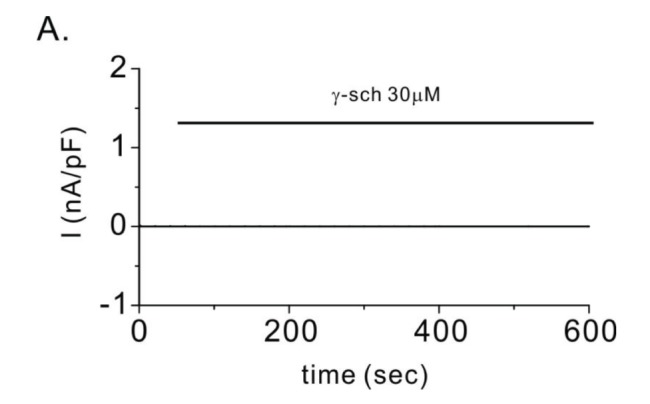
- Transient receptor potential vanilloid 3 (TRPV3) is a non-selective cation channel with modest permeability to calcium ions. It is involved in intracellular calcium signaling and is therefore important in processes such as thermal sensation, skin barrier formation, and wound healing. TRPV3 was initially proposed as a warm temperature sensor. It is activated by synthetic small-molecule chemicals and plant-derived natural compounds such as camphor and eugenol. Schisandra chinensis (Turcz.) Baill (SC) has diverse pharmacological properties including antiallergic, anti-inflammatory, and wound healing activities. It is extensively used as an oriental herbal medicine for the treatment of various diseases. In this study, we investigated whether SC fruit extracts and seed oil, as well as four compounds isolated from the fruit can activate the TRPV3 channel. By performing whole-cell patch clamp recording in HEK293T cells overexpressing TRPV3, we found that the methanolic extract of SC fruit has an agonistic effect on the TRPV3 channel. Furthermore, electrophysiological analysis revealed that γ-schisandrin, one of the isolated compounds, activated TRPV3 at a concentration of 30 µM. In addition, γ-schisandrin (~100 µM) increased cytoplasmic Ca²âº concentrations by approximately 20% in response to TRPV3 activation. This is the first report to indicate that SC extract and γ-schisandrin can modulate the TRPV3 channel. This report also suggests a mechanism by which γ-schisandrin acts as a therapeutic agent against TRPV3-related diseases.
MeSH Terms
Figure
Reference
-
1. Luo J, Hu H. Thermally activated TRPV3 channels. Curr Top Membr. 2014; 74:325–364. PMID: 25366242.
Article2. Deering-Rice CE, Mitchell VK, Romero EG, Abdel Aziz MH, Ryskamp DA, Križaj D, Gopal VR, Reilly CA. Drofenine: A 2-APB analogue with greater selectivity for human TRPV3. Pharmacol Res Perspect. 2014; 2:e00062. PMID: 25089200.3. Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006; 9:628–635. PMID: 16617338.
Article4. Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005; 307:1468–1472. PMID: 15746429.
Article5. Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007; 151:530–540. PMID: 17420775.
Article6. Cheung SY, Huang Y, Kwan HY, Chung HY, Yao X. Activation of transient receptor potential vanilloid 3 channel suppresses adipogenesis. Endocrinology. 2015; 156:2074–2086. PMID: 25774551.
Article7. Inada H, Iida T, Tominaga M. Different expression patterns of TRP genes in murine B and T lymphocytes. Biochem Biophys Res Commun. 2006; 350:762–767. PMID: 17027915.
Article8. Ueda T, Yamada T, Ugawa S, Ishida Y, Shimada S. TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem Biophys Res Commun. 2009; 383:130–134. PMID: 19336223.
Article9. Ho JC, Lee CH. TRP channels in skin: from physiological implications to clinical significances. Biophysics (Nagoya-shi). 2015; 11:17–24. PMID: 27493510.
Article10. Nilius B, Bíró T, Owsianik G. TRPV3: time to decipher a poorly understood family member! J Physiol. 2014; 592:295–304. PMID: 23836684.
Article11. Nilius B, Bíró T. TRPV3: a ‘more than skinny’ channel. Exp Dermatol. 2013; 22:447–452. PMID: 23800054.
Article12. Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, Caterina MJ, Dempsey P, Michael LE, Dlugosz AA, Andrews NC, Clapham DE, Xu H. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010; 141:331–343. PMID: 20403327.
Article13. Aijima R, Wang B, Takao T, Mihara H, Kashio M, Ohsaki Y, Zhang JQ, Mizuno A, Suzuki M, Yamashita Y, Masuko S, Goto M, Tominaga M, Kido MA. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015; 29:182–192. PMID: 25351988.
Article14. Oh EY, Jang JY, Choi YH, Choi YW, Choi BT. Inhibitory effects of 1-O-methyl-fructofuranose from Schisandra chinensis fruit on melanogenesis in B16F0 melanoma cells. J Ethnopharmacol. 2010; 132:219–224. PMID: 20723590.
Article15. Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008; 118:183–212. PMID: 18515024.
Article16. Lee SK, Kim SD, Kook M, Lee HY, Park JS, Park YH, Kang JS, Jung WJ, Choi YW, Bae YS. Therapeutic effects of α-iso-cubebenol, a natural compound isolated from the Schisandra chinensis fruit, against sepsis. Biochem Biophys Res Commun. 2012; 427:547–552. PMID: 23022181.
Article17. Lee KP, Kang S, Park SJ, Kim JM, Lee JM, Lee AY, Chung HY, Choi YW, Lee YG, Im DS. Anti-allergic effect of α-cubebenoate isolated from Schisandra chinensis using in vivo and in vitro experiments. J Ethnopharmacol. 2015; 173:361–369. PMID: 26253578.
Article18. Oh SY, Kim YH, Bae DS, Um BH, Pan CH, Kim CY, Lee HJ, Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci Biotechnol Biochem. 2010; 74:285–291. PMID: 20139628.19. Park HJ, Cho JY, Kim MK, Koh PO, Cho KW, Kim CH, Lee KS, Chung BY, Kim GS, Cho JH. Anti-obesity effect of Schisandra chinensis in 3T3-L1 cells and high fat diet-induced obese rats. Food Chem. 2012; 134:227–234.
Article20. Nam JH, Nam DY, Lee DU. Valencene from the rhizomes of cyperus rotundus inhibits skin photoaging-related ion channels and UV-induced melanogenesis in B16F10 melanoma cells. J Nat Prod. 2016; 79:1091–1096. PMID: 26967731.
Article21. Lee DU, Weon KY, Nam DY, Nam JH. Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp Dermatol. 2016; 25:977–982. PMID: 27488812.
Article22. Nam JH, Lee DU. Inhibitory effect of oleanolic acid from the rhizomes of Cyperus rotundus on transient receptor potential vanilloid 1 channel. Planta Med. 2015; 81:20–25. PMID: 25402944.
Article23. Kanatani H, Terabayashi S, Takeda S, Li W, Koike K, Nikaido T. Regio- and stereoselective 12-O-demethylation of schizandrin into gomisin T, an important intermediate to gomisin A, by Mortierella sp. (TM-I1104). Tetrahedron Lett. 2005; 46:8467–8470.
Article24. Ikeya Y, Taguchi H, Yosioka I. The constituents of schizandra chinensis BAILL. X. The structures of γ-schizandrin and four new lignans, (-)-gomisins L1 and L2, (±)-gomisin M1 and (+)-gomisin M2. Chem Pharm Bull. 1982; 30:132–139.25. Seo SM, Lee HJ, Park YK, Lee MK, Park JI, Paik KH. Lignans from the fruits of schizandra chinensis and their inhibitory effects on dopamine content in PC12 cells. Nat Prod Sci. 2004; 10:104–108.26. Ikeya Y, Tagychi H, Yosioka I. The constituents of schizandra chinensis BAILL. The structures of three new lignans, angeloylgomisin H, tigloylgomisin H and benzoylgomisin H, and the absolute structure of schizandrin. Chem Pharm Bull. 1978; 26:328–331.
Article27. Han DH, Lee JH, Kim H, Ko MK, Chae MR, Kim HK, So I, Jeon JH, Park JK, Lee SW. Effects of Schisandra chinensis extract on the contractility of corpus cavernosal smooth muscle (CSM) and Ca2+ homeostasis in CSM cells. BJU Int. 2012; 109:1404–1413. PMID: 21951618.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lignans with NADPH Oxidase 2 (NOX2)-inhibitory Activity from the Fruits of Schisandra chinensis
- Schisandrin C Protects YD-38 Cells from Cisplatin-induced Cell Death by Inhibiting Cytochrome c Release from Mitochondria
- Schisandrol A and gomisin N from Schisandra chinensis extract improve hypogonadism via anti-oxidative stress in TM3 Leydig cells
- Transient Receptor Potential Vanilloid Type-1 Channel in Cardiometabolic Protection
- Expression of vesicular glutamate transporter in transient receptor potential vanilloid 1-positive neurons in the rat trigeminal ganglion