Ann Lab Med.
2017 Jul;37(4):339-342. 10.3343/alm.2017.37.4.339.
Sigma-Metrics of Electrolyte Tests From a Recently Launched New-Generation Proficiency Testing Program of the Korean Association of Quality Assurance for Clinical Laboratory
- Affiliations
-
- 1Department of Laboratory Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 2Siemens Healthineers, Seoul, Korea.
- 3Department of Laboratory Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea.
- 4Department of Laboratory Medicine, School of Medicine, Eulji University, Daejeon, Korea.
- 5Samkwang Medical Laboratories, Seoul, Korea.
- 6Green Cross Laboratories, Yongin, Korea.
- 7Hanmi Medicare Inc., Seoul, Korea.
- 8Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. phi@catholic.ac.kr
- KMID: 2376793
- DOI: http://doi.org/10.3343/alm.2017.37.4.339
Abstract
- No abstract available.
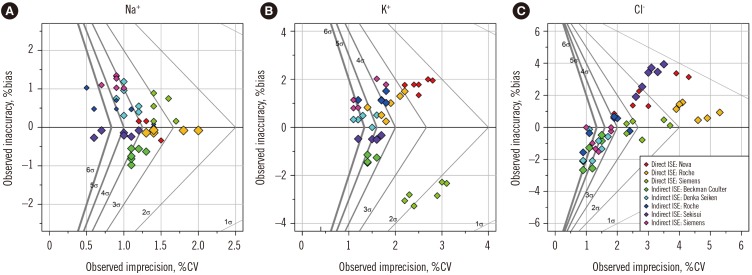
Figure
Cited by 1 articles
-
Internal Quality Control Data of Urine Reagent Strip Tests and Derivation of Control Rules Based on Sigma Metrics
Haeil Park, Younsuk Ko
Ann Lab Med. 2021;41(5):447-454. doi: 10.3343/alm.2021.41.5.447.
Reference
-
1. Korean Association of Quality Assurance for Clinical Laboratory. New Generation Proficiency Testing Program. Updated on May 2016. http://eqas.keqas.org/.2. Westgard JO, Westgard SA. Assessing quality on the Sigma scale from proficiency testing and external quality assessment surveys. Clin Chem Lab Med. 2015; 53:1531–1535. PMID: 25719323.3. Westgard JO, Westgard SA. Quality control review: implementing a scientifically based quality control system. Ann Clin Biochem. 2016; 53:32–50. PMID: 26150675.4. Revision of the “Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations-Rili-BAEK” (unauthorized translation). J Lab Med. 2015; 39:26–69.5. Orth M. Are regulation-driven performance criteria still acceptable? - The German point of view. Clin Chem Lab Med. 2015; 53:893–898. PMID: 25849796.6. Westgard SA. Utilizing global data to estimate analytical performance on the Sigma scale: A global comparative analysis of methods, instruments, and manufacturers through external quality assurance and proficiency testing programs. Clin Biochem. 2016; 49:699–707. PMID: 26948097.7. Westgard JO. Quality Requirements. Updated on Oct 2014. https://www.westgard.com/quality-requirements.htm.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Report of the Korean Association of External Quality Assessment Service on General and Specialized Coagulation Tests in Korea (2016–2019)
- Quality Assurance of Genetic Testing
- Report of the Korean Association of External Quality Assessment Service on Next-Generation Sequencing Analysis for Somatic Variants (2018–2020)
- Survey of Eight Hormone Tests Used by Clinical Laboratories in Korea
- Operational Experience of a Quality Assurance System for HPV DNA Chip Tests