Ann Lab Med.
2017 Jul;37(4):297-304. 10.3343/alm.2017.37.4.297.
Prevalence of Complement-Mediated Cell Lysis-like Gene (sicG) in Streptococcus dysgalactiae subsp. equisimilis Isolates From Japan (2014–2016)
- Affiliations
-
- 1Laboratory of Infectious Diseases, Kitasato Institute for Life Sciences, Kitasato University, Toky, Japan. taka2si@lisci.kitasato-u.ac.jp
- 2Department of Clinical Laboratory, Kitasato University Medical Center, Kitamoto, Japan.
- 3Department of Clinical Laboratory, Mishuku Hospital, Federation of National Public Service and Personnel Mutual Aid Associations, Tokyo, Japan.
- 4Division of Clinical Laboratory, Sanritsu Co., Ltd., Yachiyo, Japan.
- KMID: 2376785
- DOI: http://doi.org/10.3343/alm.2017.37.4.297
Abstract
- BACKGROUND
Streptococcus dysgalactiae subsp. equisimilis (SDSE; a β-hemolytic streptococcus of human or animal origin) infections are emerging worldwide. We evaluated the clonal distribution of complement-mediated cell lysis-like gene (sicG) among SDSE isolates from three central prefectures of Japan.
METHODS
Group G/C β-hemolytic streptococci were collected from three institutions from April 2014 to March 2016. Fifty-five strains (52 from humans and three from animals) were identified as SDSE on the basis of 16S rRNA sequencing data.; they were obtained from 25 sterile (blood, joint fluid, and cerebrospinal fluid) and 30 non-sterile (skin-, respiratory tract-, and genitourinary tract-origin) samples. emm genotyping, multilocus sequence typing, sicG amplification/sequencing, and random amplified polymorphic DNA (RAPD) analysis of sicG-positive strains were performed.
RESULTS
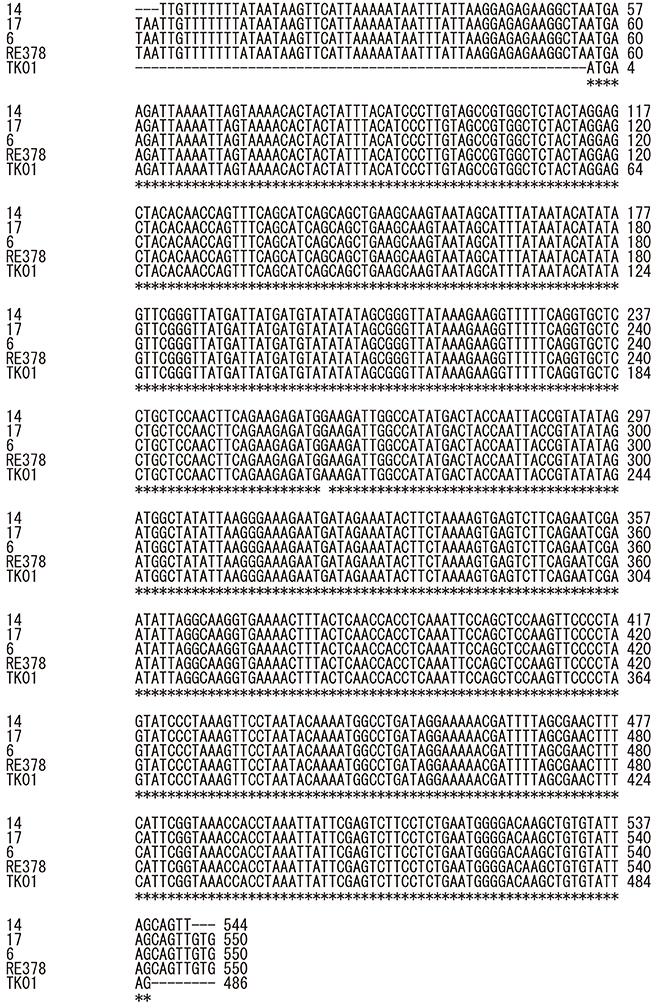
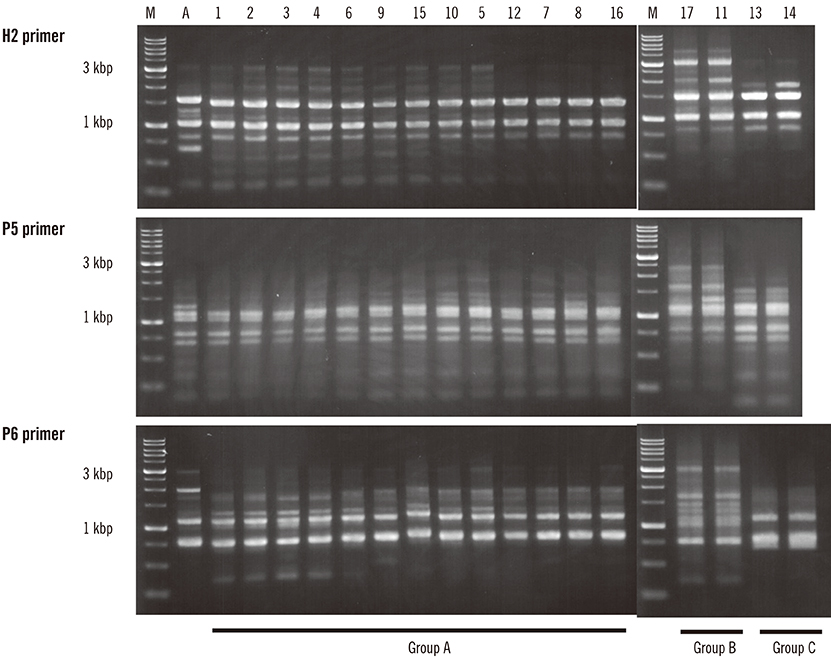
sicG was detected in 30.9% of the isolates (16 human and one canine) and the genes from the 16 human samples (blood, 10; open pus, 3; sputum, 2; throat swab, 1) and one canine sample (open pus) showed the same sequence pattern. All sicG-harboring isolates belonged to clonal complex (CC) 17, and the most prevalent emm type was stG6792 (82.4%). There was a significant association between sicG presence and the development of skin/soft tissue infections. CC17 isolates with sicG could be divided into three subtypes by RAPD analysis.
CONCLUSIONS
CC17 SDSE harboring sicG might have spread into three closely-related prefectures in central Japan during 2014-2016. Clonal analysis of isolates from other areas might be needed to monitor potentially virulent strains in humans and animals.
Keyword
MeSH Terms
Figure
Reference
-
1. Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996; 46:774–781.2. Broyles LN, Van Beneden C, Beall B, Facklam R, Shewmaker PL, Malpiedi P, et al. Population-based study of invasive disease due to β-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009; 48:706–712.
Article3. Cohen-Poradosu R, Jaffe J, Lavi D, Grisariu-Greenzaid S, Nir-Paz R, Valinsky L, et al. Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis. 2004; 10:1455–1460.
Article4. Liao CH, Liu LC, Huang YT, Teng LJ, Hsueh PR. Bacteremia caused by group G streptococci, Taiwan. Emerg Infect Dis. 2008; 14:837–840.
Article5. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med. 2002; 112:622–626.
Article6. Takahashi T, Ubukata K, Watanabe H. Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother. 2011; 17:1–10.7. Yamaoka S, Ogihara T, Yasui M, Hasegawa M, Hira S, Oue S, et al. Neonatal streptococcal toxic shock syndrome caused by Streptococcus dysgalactiae subsp. equisimilis. Pediatr Infect Dis J. 2010; 29:979–981.8. Bramhachari PV, Kaul SY, McMillan DJ, Shaila MS, Karmarkar MG, Sriprakash KS. Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J Med Microbiol. 2010; 59:220–223.9. Takahashi T, Sunaoshi K, Sunakawa K, Fujishima S, Watanabe H, Ubukata K. Clinical aspects of invasive infections with Streptococcus dysgalactiae ssp. equisimilis in Japan: differences with respect to Streptococcus pyogenes and Streptococcus agalactiae infections. Clin Microbiol Infect. 2010; 16:1097–1103.10. Åkesson P, Sjöholm AG, Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996; 271:1081–1088.11. Fernie-King BA, Seilly DJ, Willers C, Würzner R, Davies A, Lachmann PJ. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology. 2001; 103:390–398.
Article12. Minami M, Ichikawa M, Matsui H, Hata N, Wakiyama N, Matsumoto M, et al. Prevalence of a streptococcal inhibitor of a complement-mediated cell lysis-like gene (sicG) in Streptococcus dysgalactiae subsp. equisimilis. Curr Microbiol. 2011; 62:884–887.13. Smyth D, Cameron A, Davies MR, McNeilly C, Hafner L, Sriprakash KS, et al. DrsG from Streptococcus dysgalactiae subsp. equisimilis inhibits the antimicrobial peptide LL-37. Infect Immun. 2014; 82:2337–2344.14. Fujita T, Horiuchi A, Ogawa M, Yoshida H, Hirose Y, Nagano N, et al. Genetic diversity in Streptococcus dysgalactiae subsp. equisimilis isolates from patients with invasive and noninvasive infections in a Japanese university hospital (2014–2015). Jpn J Infect Dis. 2017; 70:100–104.15. Streptococcus dysgalactiae subsp. equisimilis. Vandamme et al. emend. Vieira et al. (ATCC® 12394™). Accessed on September 8, 2015. http://www.atcc.org/products/all/12394.aspx.16. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second Informational supplement, M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute;2012.17. McMillan DJ, Bessen DE, Pinho M, Ford C, Hall GS, Melo-Cristino J, et al. Population genetics of Streptococcus dysgalactiae subspecies equisimilis reveals widely dispersed clones and extensive recombination. PLoS One. 2010; 5:e11741.18. Bert F, Picard B, Branger C, Lambert-Zechovsky N. Analysis of genetic relationships among strains of groups A, C and G streptococci by random amplified polymorphic DNA analysis. J Med Microbiol. 1996; 45:278–284.
Article19. Haenni M, Saras E, Bertin S, Leblond P, Madec JY, Payot S. Diversity and mobility of integrative and conjugative elements in bovine isolates of Streptococcus agalactiae, S. dysgalactiae subsp. dysgalactiae, and S. uberis. Appl Environ Microbiol. 2010; 76:7957–7965.20. Malhotra-Kumar S, Lammens C, Piessens J, Goossens H. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob Agents Chemother. 2005; 49:4798–4800.
Article21. Lo HH, Cheng WS. Distribution of virulence factors and association with emm polymorphism or isolation site among beta-hemolytic group G Streptococcus dysgalactiae subspecies equisimilis. APMIS. 2015; 123:45–52.22. Oppegaard O, Mylvaganam H, Skrede S, Langeland N, Kittang BR. Sequence diversity of sicG among group C and G Streptococcus dysgalactiae subspecies equisimilis isolates associated with human infections in western Norway. Eur J Clin Microbiol Infect Dis. 2014; 33:273–277.23. Davies MR, McMillan DJ, Beiko RG, Barroso V, Geffers R, Sriprakash KS, et al. Virulence profiling of Streptococcus dysgalactiae subspecies equisimilis isolated from infected humans reveals 2 distinct genetic lineages that do not segregate with their phenotypes or propensity to cause diseases. Clin Infect Dis. 2007; 44:1442–1454.24. Mejia LM, Stockbauer KE, Pan X, Cravioto A, Musser JM. Characterization of group A Streptococcus strains recovered from Mexican children with pharyngitis by automated DNA sequencing of virulence-related genes: unexpectedly large variation in the gene (sic) encoding a complement-inhibiting protein. J Clin Microbiol. 1997; 35:3220–3224.25. Schrieber L, Towers R, Muscatello G, Speare R. Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses Public Health. 2014; 61:145–148.26. Centers for Disease Control and Prevention. One Health. Updated on January 25, 2017. https://www.cdc.gov/onehealth/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Characteristics of Streptococcus dysgalactiae subsp. equisimilis Isolates Causing Repetitive vs Single Infections
- Molecular Epidemiological Features and Antibiotic Susceptibility Patterns of Streptococcus dysgalactiae subsp. equisimilis Isolates from Korea and Japan
- A Case of Neonatal Meningitis Caused by Streptococcus dysgalactiae subspecies dysgalactiae and Herpes Simplex Virus
- Identification of streptococcus dysgalactiae subsp. equisimilis from septic knee by 16S rRNA gene sequencing
- Pathologic and molecular characterization of Streptococcus dysgalactiae subsp. equisimilis infection in neonatal piglets