Diabetes Metab J.
2017 Apr;41(2):121-127. 10.4093/dmj.2017.41.2.121.
Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density?
- Affiliations
-
- 1Obesity-Diabetes Advanced Research Center, Yeungnam University College of Medicine, Daegu, Korea. jykim@ynu.ac.kr
- KMID: 2376774
- DOI: http://doi.org/10.4093/dmj.2017.41.2.121
Abstract
- BACKGROUND
The proportion of saturated fatty acids/unsaturated fatty acids in the diet seems to act as a physiological regulation on obesity, cardiovascular diseases, and diabetes. Differently composed fatty acid diets may induce satiety of the hypothalamus in different ways. However, the direct effect of the different fatty acid diets on satiety in the hypothalamus is not clear.
METHODS
Three experiments in mice were conducted to determine whether: different compositions of fatty acids affects gene mRNA expression of the hypothalamus over time; different types of fatty acids administered into the stomach directly affect gene mRNA expression of the hypothalamus; and fat composition changes in the diet affects gene mRNA expression of the hypothalamus.
RESULTS
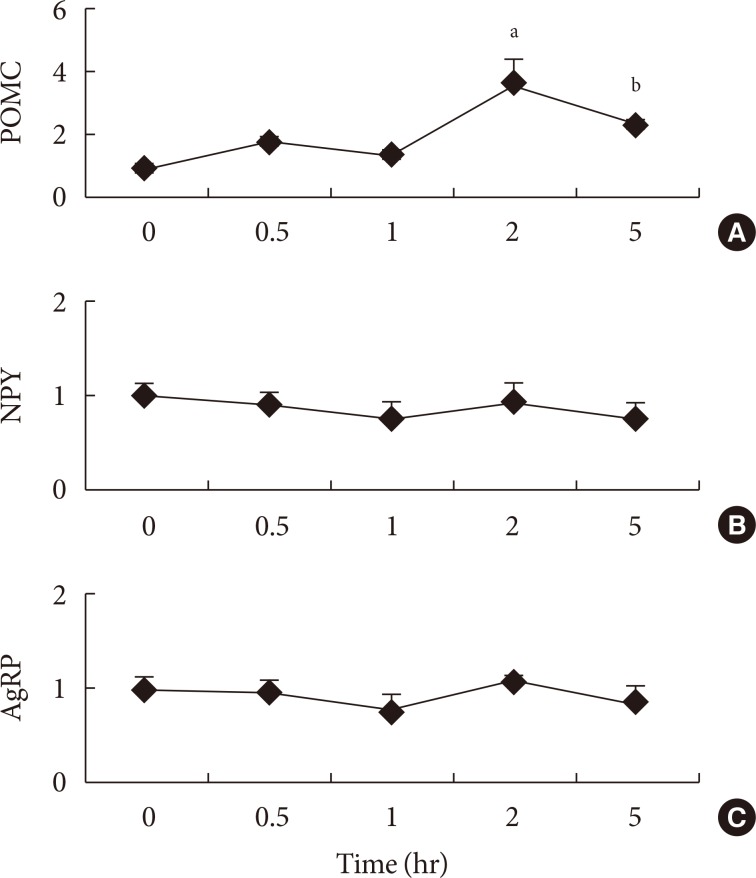
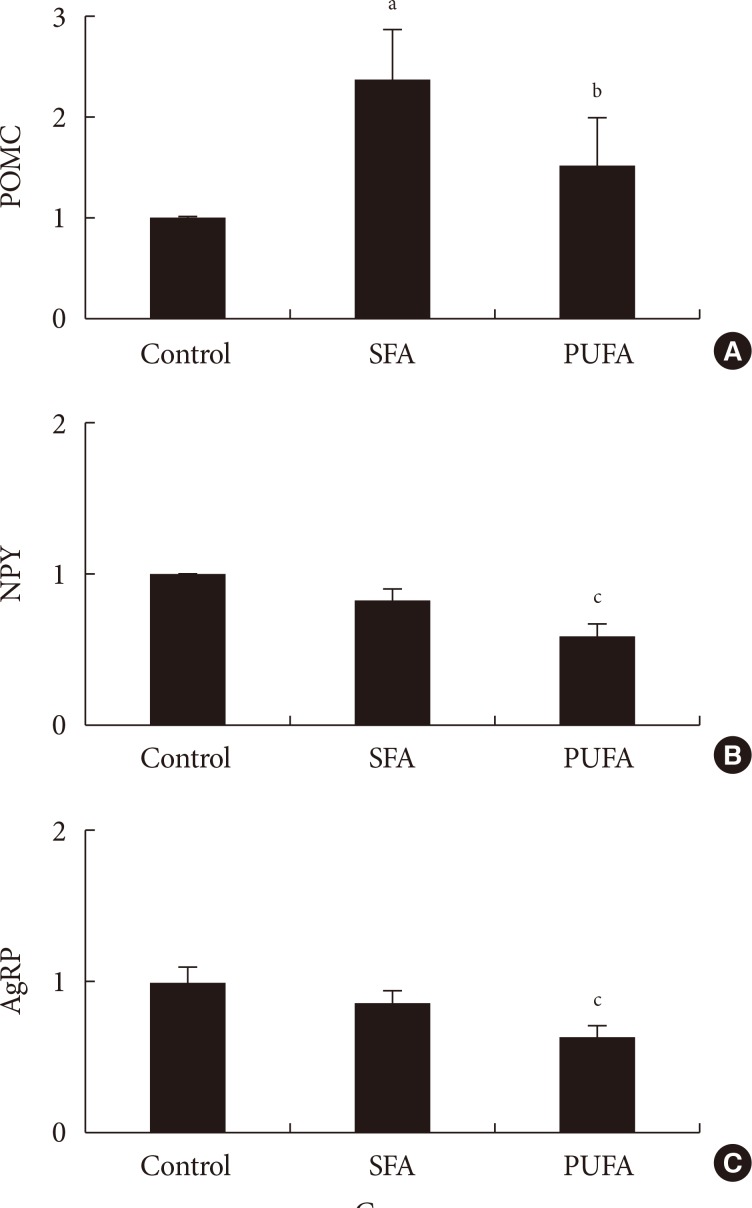
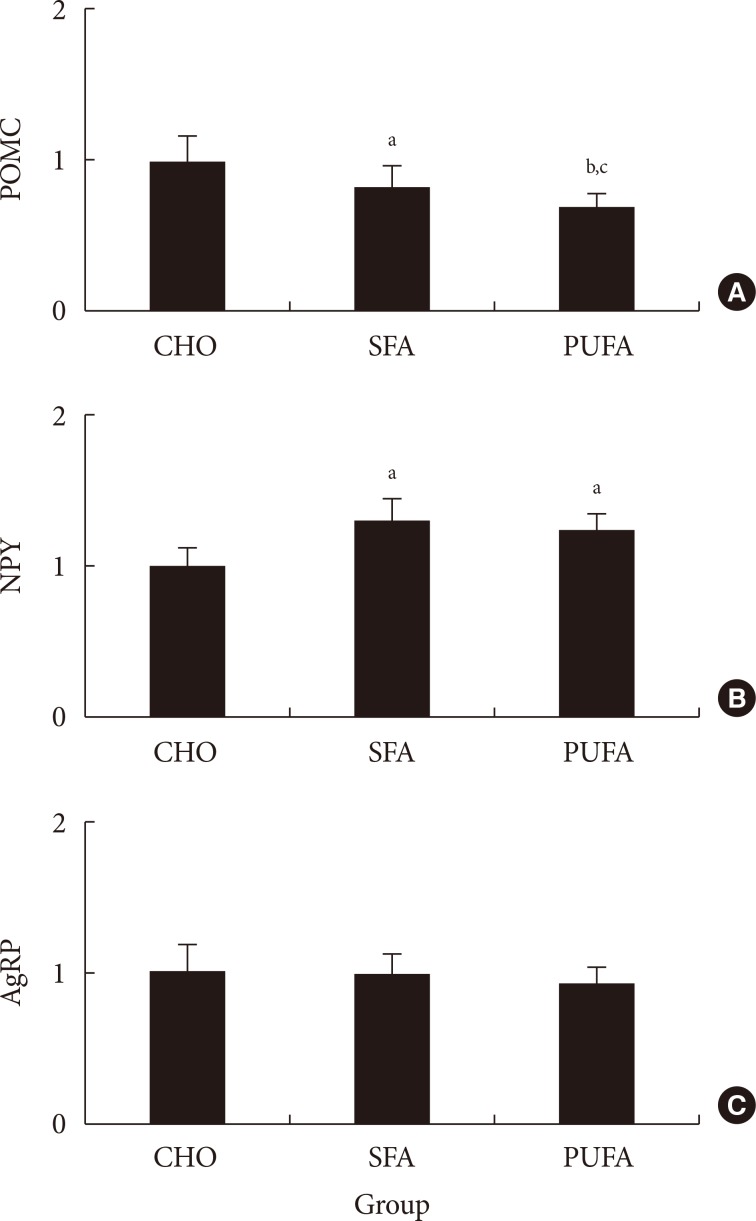
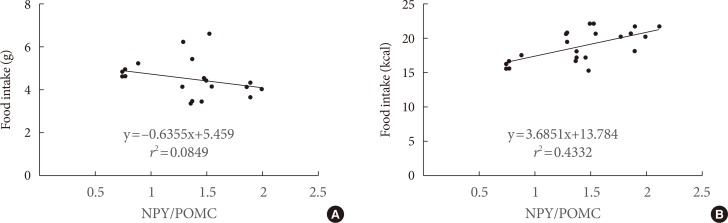
The type of fat in cases of purified fatty acid administration directly into the stomach may cause changes of gene expressions in the hypothalamus. Gene expression by dietary fat may be regulated by calorie amount ingested rather than weight amount or type of fat.
CONCLUSION
Therefore, the calorie density factor of the diet in regulating hypothalamic gene in food intake may be detrimental, although the possibility of type of fat cannot be ruled out.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Letter: Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density? (
Diabetes Metab J 2017;41:121-7)
Bo Kyung Koo
Diabetes Metab J. 2017;41(3):223-224. doi: 10.4093/dmj.2017.41.3.223.
Reference
-
1. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999; 282:1523–1529. PMID: 10546691.
Article2. Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr. 2009; 48(Suppl 1):S31–S38. PMID: 19214056.
Article3. Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004; 83:573–578. PMID: 15621062.
Article4. Kim JY, Nolte LA, Hansen PA, Han DH, Ferguson K, Thompson PA, Holloszy JO. High-fat diet-induced muscle insulin resistance: relationship to visceral fat mass. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R2057–R2065. PMID: 11080069.
Article5. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015; (6):CD011737. PMID: 26068959.
Article6. Schwab U, Lauritzen L, Tholstrup T, Haldorssoni T, Riserus U, Uusitupa M, Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr Res. 2014; 58:25145.
Article7. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010; 7:e1000252. PMID: 20351774.
Article8. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, Anand SS. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015; 351:h3978. PMID: 26268692.
Article9. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010; 91:535–546. PMID: 20071648.
Article10. Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006; 98:27i–33i.
Article11. Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006; 83(6):Suppl. 1520S–1525S. PMID: 16841862.
Article12. Kim YS, Xun P, Iribarren C, Van Horn L, Steffen L, Daviglus ML, Siscovick D, Liu K, He K. Intake of fish and long-chain omega-3 polyunsaturated fatty acids and incidence of metabolic syndrome among American young adults: a 25-year follow-up study. Eur J Nutr. 2016; 55:1707–1716. PMID: 26816031.
Article13. Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999; 70(3 Suppl):560S–569S. PMID: 10479232.
Article14. Winnicki M, Somers VK, Accurso V, Phillips BG, Puato M, Palatini P, Pauletto P. Fish-rich diet, leptin, and body mass. Circulation. 2002; 106:289–291. PMID: 12119240.
Article15. Ukropec J, Reseland JE, Gasperikova D, Demcakova E, Madsen L, Berge RK, Rustan AC, Klimes I, Drevon CA, Sebokova E. The hypotriglyceridemic effect of dietary n-3 FA is associated with increased beta-oxidation and reduced leptin expression. Lipids. 2003; 38:1023–1029. PMID: 14669966.16. Fan C, Liu X, Shen W, Deckelbaum RJ, Qi K. The regulation of leptin, leptin receptor and pro-opiomelanocortin expression by N-3 PUFAs in diet-induced obese mice is not related to the methylation of their promoters. Nutr Metab (Lond). 2011; 8:31. PMID: 21609458.
Article17. Bouret SG. Leptin, nutrition, and the programming of hypothalamic feeding circuits. Nestle Nutr Workshop Ser Pediatr Program. 2010; 65:25–35.
Article18. Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000; 404:644–651. PMID: 10766251.
Article19. Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science. 2005; 307:375–379. PMID: 15662002.
Article20. Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007; 129:251–262. PMID: 17448988.
Article21. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000; 404:661–671. PMID: 10766253.
Article22. Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology. 2003; 144:5172–5178. PMID: 12970158.
Article23. Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004; 53:2521–2528. PMID: 15448079.24. Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005; 360:2227–2235. PMID: 16321792.
Article25. Hoefel AL, Hansen F, Rosa PD, Assis AM, Silveira SL, Denardin CC, Pettenuzzo L, Augusti PR, Somacal S, Emanuelli T, Perry ML, Wannmacher CM. The effects of hypercaloric diets on glucose homeostasis in the rat: influence of saturated and monounsaturated dietary lipids. Cell Biochem Funct. 2011; 29:569–576. PMID: 21837644.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density? (Diabetes Metab J 2017;41:121-7)
- Response: Regulating Hypothalamus Gene Expression in Food Intake: Dietary Composition or Calorie Density? (Diabetes Metab J 2017;41:121-7)
- Comparison of Calorie Intake and Satiety Rate by Different Energy Density Level of Kimbab
- Analysis of the Relationship between Dietary Fiber Intake & Food Habits in the Korean Adult Population: Using the 2001 Korean National Health and Nutrition Survey Data and the Newly Established Dietary Fiber Database
- Effects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice