Korean J Pain.
2017 Apr;30(2):98-103. 10.3344/kjp.2017.30.2.98.
Antinociceptive effect of intrathecal sec-O-glucosylhamaudol on the formalin-induced pain in rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, School of Medicine, Chosun University, Gwangju, Korea. mdmole@chosun.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Chosun University Hospital, Gwangju, Korea.
- 3Department of Anesthesiology and Pain Medicine, Medical School, Chonnam National University, Gwangju, Korea.
- 4Department of Premedics, School of Medicine, Chosun University, Gwangju, Korea.
- KMID: 2376045
- DOI: http://doi.org/10.3344/kjp.2017.30.2.98
Abstract
- BACKGROUND
The root of Peucedanum japonicum Thunb., a perennial herb found in Japan, the Philippines, China, and Korea, is used as an analgesic. In a previous study, sec-O-glucosylhamaudol (SOG) showed an analgesic effect. This study was performed to examine the antinociceptive effect of intrathecal SOG in the formalin test.
METHODS
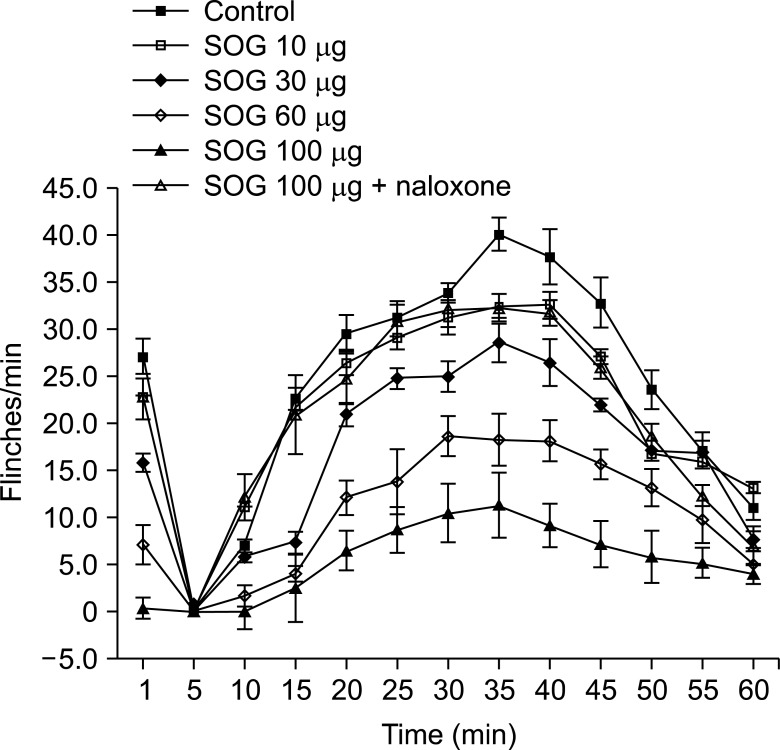
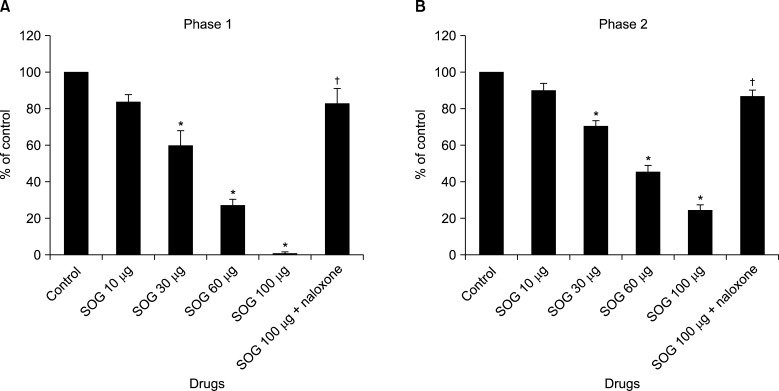
Male Sprague-Dawley rats were implanted with an intrathecal catheter. Rats were randomly treated with a vehicle and SOG (10 µg, 30 µg, 60 µg, and 100 µg) before formalin injection. Five percent formalin was injected into the hind-paw, and a biphasic reaction followed, consisting of flinching and licking behaviors (phase 1, 0-10 min; phase 2, 10-60 min). Naloxone was injected 10 min before administration of SOG 100 µg to evaluate the involvement of SOG with an opioid receptor. Dose-responsiveness and ED50 values were calculated.
RESULTS
Intrathecal SOG showed a significant reduction of the flinching responses at both phases in a dose-dependent manner. Significant effects were showed from the dose of 30 µg and maximum effects were achieved at a dose of 100 µg in both phases. The ED50 value (95% confidence intervals) of intrathecal SOG was 30.3 (25.8-35.5) µg during phase 1, and 48.0 (41.4-55.7) during phase 2. The antinociceptive effects of SOG (100 µg) were significantly reverted at both phases of the formalin test by naloxone.
CONCLUSIONS
These results demonstrate that intrathecal SOG has a very strong antinociceptive effect in the formalin test and it seems the effect is related to an opioid receptor.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Effect of sec-O-glucosylhamaudol on mechanical allodynia in a rat model of postoperative pain
Gi-Ho Koh, Hyun Song, Sang Hun Kim, Myung Ha Yoon, Kyung Joon Lim, Seon-Hee Oh, Ki Tae Jung
Korean J Pain. 2019;32(2):87-96. doi: 10.3344/kjp.2019.32.2.87.Sec-O-glucosylhamaudol mitigates inflammatory processes and autophagy via p38/JNK MAPK signaling in a rat neuropathic pain model
Seon Hee Oh, Suk Whee Kim, Dong Joon Kim, Sang Hun Kim, Kyung Joon Lim, Kichang Lee, Ki Tae Jung
Korean J Pain. 2021;34(4):405-416. doi: 10.3344/kjp.2021.34.4.405.
Reference
-
1. McCurdy CR, Scully SS. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005; 78:476–484. PMID: 16216276.
Article2. Ikeshiro Y, Mase I, Tomita Y. Dihydropyranocoumarins from roots of Peucedanum japonicum. Phytochemistry. 1992; 31:4303–4306.
Article3. Chen IS, Chang CT, Sheen WS, Teng CM, Tsai IL, Duh CY, et al. Coumarins and antiplatelet aggregation constituents from Formosan Peucedanum japonicum. Phytochemistry. 1996; 41:525–530. PMID: 8821432.
Article4. Hisamoto M, Kikuzaki H, Ohigashi H, Nakatani N. Antioxidant compounds from the leaves of Peucedanum japonicum thunb. J Agric Food Chem. 2003; 51:5255–5261. PMID: 12926867.
Article5. Zimecki M, Artym J, Cisowski W, Mazol I, Włodarczyk M, Gleńsk M. Immunomodulatory and anti-inflammatory activity of selected osthole derivatives. Z Naturforsch C. 2009; 64:361–368. PMID: 19678539.
Article6. Zheng M, Jin W, Son KH, Chang HW, Kim HP, Bae KH, et al. The constituents isolated from Peucedanum japonicum Thunb. and their cyclooxygenase (COX) inhibitory activity. Korean J Med Crop Sci. 2005; 13:75–79.7. Okuyama E, Hasegawa T, Matsushita T, Fujimoto H, Ishibashi M, Yamazaki M. Analgesic components of saposhnikovia root (Saposhnikovia divaricata). Chem Pharm Bull (Tokyo). 2001; 49:154–160. PMID: 11217101.
Article8. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–110. PMID: 6877845.
Article9. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976; 17:1031–1036. PMID: 14677603.
Article10. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996; 64:345–355. PMID: 8740613.
Article11. Tallarida RJ. Drug synergism and dose-effect data analysis. Boca Raton (FL): Chapman & Hall/CRC;2000. p. 57–71.12. Sarkhail P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: a review. J Ethnopharmacol. 2014; 156:235–270. PMID: 25193684.
Article13. Sasaki H, Taguchi H, Endo T, Yosioka I. The constituents of Ledebouriella seseloides Wolff. I. structures of three new chromones. Chem Pharm Bull (Tokyo). 1982; 30:3555–3562.
Article14. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987; 30:103–114. PMID: 3614974.
Article15. Yoon MH, Park KD, Lee HG, Kim WM, An TH, Kim YO, et al. Additive antinociception between intrathecal sildenafil and morphine in the rat formalin test. J Korean Med Sci. 2008; 23:1033–1038. PMID: 19119449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- Effect of Serotonergic Receptors on the Antinociception of Intrathecal Gabapentin in the Formalin Test of Rats
- Antinociceptive Effects of Intrathecal Melatonin on Formalin: and Thermal-induced Pain in Rats
- The Antinociceptive Effect of Intrathecal Anticholinesterase on the Formalin Test in Rats
- Antinociceptive drug interaction between intrathecal vitamin E and gabapentin in the rat formalin test