J Clin Neurol.
2017 Apr;13(2):155-161. 10.3988/jcn.2017.13.2.155.
Rituximab Treatment for Idiopathic Hypertrophic Pachymeningitis
- Affiliations
-
- 1Department of Neurology, Seoul National University Hospital, Seoul, Korea. slee@snuh.org
- KMID: 2376016
- DOI: http://doi.org/10.3988/jcn.2017.13.2.155
Abstract
- BACKGROUND AND PURPOSE
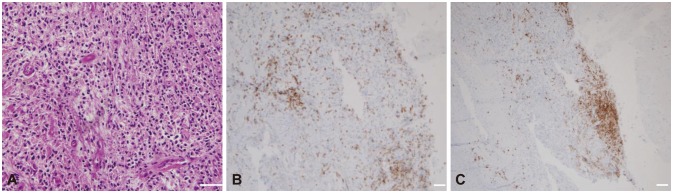
Hypertrophic pachymeningitis (HP) is a rare disease caused by autoimmunity in the meninx that causes various neurologic symptoms, including headache, seizures, weakness, paresthesia, and cranial nerve palsies. Although the first-line therapy for HP is steroids, many HP cases are refractory to steroids or recur when the steroids are tapered. Here we report three HP cases that were successfully treated with rituximab (RTX).
METHODS
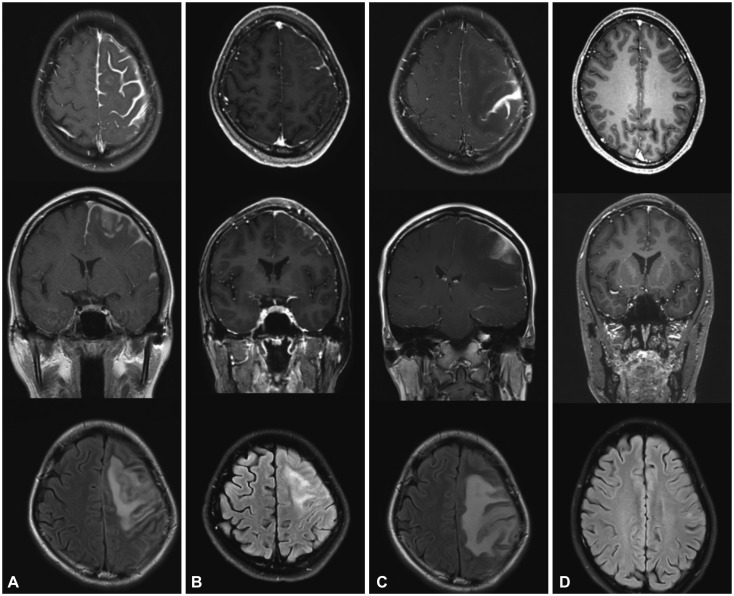
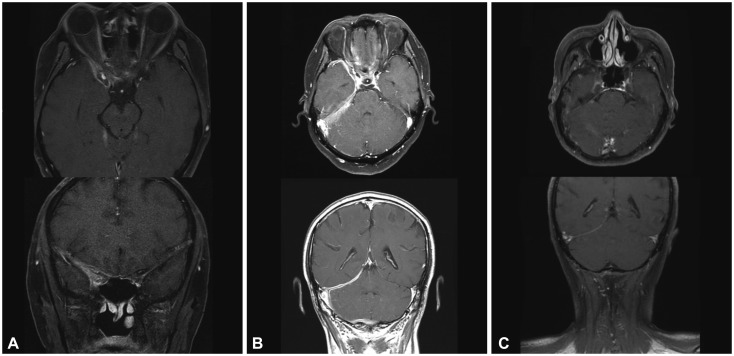
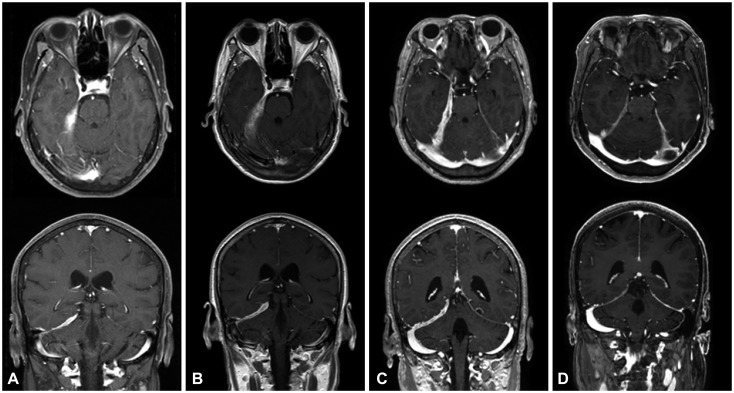
From an institutional cohort recruited from April 2012 to July 2016, three HP cases that were identified to be steroid-refractory were treated with RTX (four weekly doses of 375 mg/m²). Clinical improvement was assessed by the number of relapses of any neurologic symptom and the largest dural thickness in MRI.
RESULTS
All three patients were recurrence-free of neurologic symptoms and exhibited prominent decreases in the dural thickness after RTX treatment. No adverse events were observed in the patients.
CONCLUSIONS
We suggest RTX as a second-line therapy for steroid-refractory HP. Further studies are warranted to confirm this observation in a larger population and to consider RTX as a first-line therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Naffziger HC, Stern WE. Chronic pachymeningitis; report of a case and review of the literature. Arch Neurol Psychiatry. 1949; 62:383–411.2. Masson C, Hénin D, Hauw JJ, Rey A, Raverdy P, Masson M. Cranial pachymeningitis of unknown origin: a study of seven cases. Neurology. 1993; 43:1329–1334. PMID: 8327133.3. Martin N, Masson C, Hénin D, Mompoint D, Marsault C, Nahum H. Idiopathic cranial pachymeningitis. Assessment with CT and MR imaging. In : Nadjmi N, editor. Imaging of brain metabolism spine and cord interventional neuroradiology free communications: XVth congress of the European Society of Neuroradiology Würzburg, September 13th-17th. Berlin: Springer;1989. p. 492.4. Mamelak AN, Kelly WM, Davis RL, Rosenblum ML. Idiopathic hypertrophic cranial pachymeningitis. Report of three cases. J Neurosurg. 1993; 79:270–276. PMID: 8331412.5. Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004; 62:686–694. PMID: 15007115.
Article6. Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013; 92:206–216. PMID: 23793110.
Article7. Riku S, Kato S. Idiopathic hypertrophic pachymeningitis. Neuropathology. 2003; 23:335–344. PMID: 14719551.
Article8. Popkirov S, Kowalski T, Schlegel U, Skodda S. Immunoglobulin-G4-related hypertrophic pachymeningitis with antineutrophil cytoplasmatic antibodies effectively treated with rituximab. J Clin Neurosci. 2015; 22:1038–1040. PMID: 25861887.
Article9. Sharma A, Kumar S, Wanchu A, Lal V, Singh R, Gupta V, et al. Successful treatment of hypertrophic pachymeningitis in refractory Wegener's granulomatosis with rituximab. Clin Rheumatol. 2010; 29:107–110. PMID: 19802640.
Article10. Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore). 2012; 91:57–66. PMID: 22210556.11. Liao B, Kamiya-Matsuoka C, Fang X, Smith RG. Refractory IgG4-related intracranial hypertrophic pachymeningitis responded to rituximab. Neurol Neuroimmunol Neuroinflamm. 2014; 1:e41. PMID: 25364775.
Article12. Bosman T, Simonin C, Launay D, Caron S, Destée A, Defebvre L. Idiopathic hypertrophic cranial pachymeningitis treated by oral methotrexate: a case report and review of literature. Rheumatol Int. 2008; 28:713–718. PMID: 18094971.
Article13. Choi IS, Park SC, Jung YK, Lee SS. Combined therapy of corticosteroid and azathioprine in hypertrophic cranial pachymeningitis. Eur Neurol. 2000; 44:193–198. PMID: 11096216.
Article14. Hyun JW, Kim SH, Yoo H, Hong EK, Huh SY, Kim HJ. Steroid-resistant relapsing IgG4-related pachymeningitis treated with methotrexate. JAMA Neurol. 2014; 71:222–225. PMID: 24322883.
Article15. Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003; 22:7359–7368. PMID: 14576843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid Recurrence of Spinal Idiopathic Hypertrophic Pachymeningitis: A Case Report
- Idiopathic Hypertrophic Cranial Pachymeningitis: Case Report
- An Idiopathic Hypertrophic Tentorial Pachymeningitis Presented as an Alternating Recurrent Painful Ophthalmoplegia
- Idiopathic Hypertrophic Pachymeningitis in the Craniocervical Junction
- A case of idiopathic hypertrophic pachymeningitis presented with seizures