J Clin Neurol.
2017 Apr;13(2):129-137. 10.3988/jcn.2017.13.2.129.
Role of Perfusion-Weighted Imaging in a Diffusion-Weighted-Imaging-Negative Transient Ischemic Attack
- Affiliations
-
- 1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sukwon@amc.seoul.kr
- 2Department of Neurology, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2376013
- DOI: http://doi.org/10.3988/jcn.2017.13.2.129
Abstract
- BACKGROUND AND PURPOSE
The absence of acute ischemic lesions in diffusion-weighted imaging (DWI) in transient ischemic attack (TIA) patients makes it difficult to diagnose the true vascular etiologies. Among patients with DWI-negative TIA, we investigated whether the presence of a perfusion-weighted imaging (PWI) abnormality implied a true vascular event by identifying new acute ischemic lesions in follow-up magnetic resonance imaging (MRI) in areas corresponding to the initial PWI abnormality.
METHODS
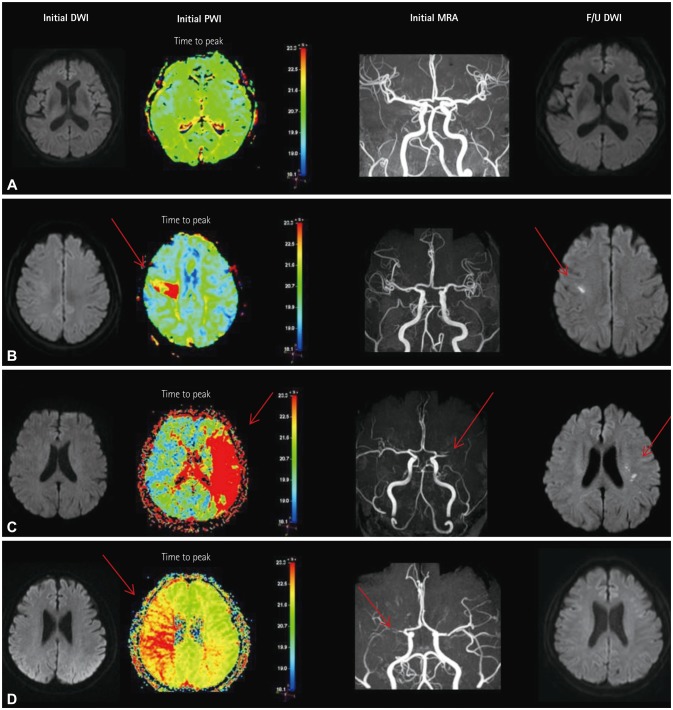
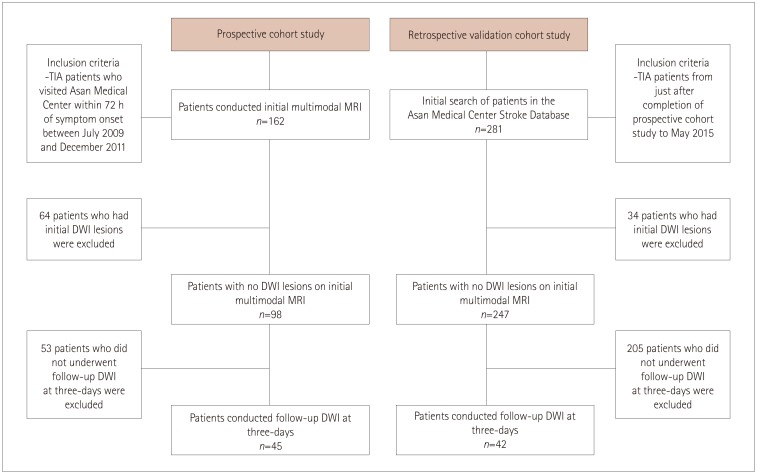
The included patients underwent DWI and PWI within 72 hours of TIA and also follow-up DWI at 3 days after the initial MRI. These patients had visited the emergency room between July 2009 and May 2015. Patients who demonstrated initial DWI lesions were excluded. The initial PWI abnormalities in the corresponding vascular territory were visually classified into three patterns: no abnormality, focal abnormality, and territorial abnormality.
RESULTS
No DWI lesions were evident in initial MRI in 345 of the 443 TIA patients. Follow-up DWI was applied to 87 of these 345 DWI-negative TIA patients. Initial PWI abnormalities were significantly associated with follow-up DWI abnormalities: 8 of 43 patients with no PWI abnormalities (18.6%) had new ischemic lesions, whereas 13 of 16 patients with focal perfusion abnormalities (81.2%) had new ischemic lesions in the areas of initial PWI abnormalities [odds ratio (OR)=15.1, 95% confidence interval (CI)=3.6-62.9], and 14 of 28 patients with territorial perfusion abnormalities (50%) had new lesions (OR=3.7, 95% CI=1.2-11.5).
CONCLUSIONS
PWI is useful in defining whether or not the transient neurological symptoms in DWI-negative TIA are true vascular events, and will help to improve the understanding of the pathomechanism of TIA.
MeSH Terms
Figure
Reference
-
1. Zaharchuk G, Olivot JM, Fischbein NJ, Bammer R, Straka M, Kleinman JT, et al. Arterial spin labeling imaging findings in transient ischemic attack patients: comparison with diffusion- and bolus perfusion-weighted imaging. Cerebrovasc Dis. 2012; 34:221–228. PMID: 23006669.
Article2. Chang JY, Kim DH, Chung JH, Yum KS, Hong JH, Han MK. Hospital-based prospective registration of acute transient ischemic attack and noncerebrovascular events in Korea. J Stroke Cerebrovasc Dis. 2015; 24:1803–1810. PMID: 26139456.
Article3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000; 284:2901–2906. PMID: 11147987.
Article4. Purroy F, Begué R, Quílez A, Piñol-Ripoll G, Sanahuja J, Brieva L, et al. The California, ABCD, and unified ABCD2 risk scores and the presence of acute ischemic lesions on diffusion-weighted imaging in TIA patients. Stroke. 2009; 40:2229–2232. PMID: 19372450.
Article5. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587. PMID: 7477192.6. Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke. 1999; 30:1174–1180. PMID: 10356095.
Article7. Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007; 370:1432–1442. PMID: 17928046.
Article8. Nah HW, Kwon SU, Kang DW, Lee DH, Kim JS. Diagnostic and prognostic value of multimodal MRI in transient ischemic attack. Int J Stroke. 2014; 9:895–901. PMID: 24256197.
Article9. Lamy C, Oppenheim C, Calvet D, Domigo V, Naggara O, Méder JL, et al. Diffusion-weighted MR imaging in transient ischaemic attacks. Eur Radiol. 2006; 16:1090–1095. PMID: 16395534.
Article10. Kim BJ, Kang HG, Kim HJ, Ahn SH, Kim NY, Warach S, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke. 2014; 16:131–145. PMID: 25328872.
Article11. Qiao XJ, Salamon N, Wang DJ, He R, Linetsky M, Ellingson BM, et al. Perfusion deficits detected by arterial spin-labeling in patients with TIA with negative diffusion and vascular imaging. AJNR Am J Neuroradiol. 2013; 34:2125–2130. PMID: 23721895.
Article12. Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003; 34:1425–1430. PMID: 12738899.
Article13. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994; 36:557–565. PMID: 7944288.
Article14. Røhl L, Ostergaard L, Simonsen CZ, Vestergaard-Poulsen P, Andersen G, Sakoh M, et al. Viability thresholds of ischemic penumbra of hyperacute stroke defined by perfusion-weighted MRI and apparent diffusion coefficient. Stroke. 2001; 32:1140–1146. PMID: 11340223.
Article15. Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002; 105:656–662. PMID: 11827935.
Article16. Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994; 25:1847–1853. discussion 1853-1854. PMID: 8073468.
Article17. del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991; 22:1276–1283. PMID: 1926239.
Article18. del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003; 23:879–894. PMID: 12902832.
Article19. del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000; 98:73–81.
Article21. Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003; 54:66–74. PMID: 12838521.
Article22. Greif DM, Eichmann A. Vascular biology: brain vessels squeezed to death. Nature. 2014; 508:50–51. PMID: 24670635.23. Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014; 508:55–60. PMID: 24670647.
Article24. Garcia JH, Kamijyo Y. Cerebral infarction. Evolution of histopathological changes after occlusion of a middle cerebral artery in primates. J Neuropathol Exp Neurol. 1974; 33:408–421. PMID: 4209682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Early Perfusion Weighted MRI for the Diagnosis of TIA
- Small Vessel Transient Ischemic Attack and Lacunar Infarction Detected with Perfusion-Weighted MRI
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Diagnosis and Treatment of Acute Ischemic Stroke Guided by Stroke MRI
- Reversal of Diffusion-Weighted Imaging Hyperintensities of the Pons after Endovascular Reperfusion for Proximal Extracranial Vertebral Artery Occlusion