Tuberc Respir Dis.
2017 Apr;80(2):179-186. 10.4046/trd.2017.80.2.179.
Keratinization of Lung Squamous Cell Carcinoma Is Associated with Poor Clinical Outcome
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. narae97@yuhs.ac
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2375987
- DOI: http://doi.org/10.4046/trd.2017.80.2.179
Abstract
- BACKGROUND
Although the World Health Organization (WHO) classification of lung squamous cell carcinoma (SCC) was revised in 2015, its clinical implications for lung SCC subsets remain unclear. We investigated whether the morphologic characteristics of lung SCC, including keratinization, were associated with clinical parameters and clinical outcome of patients.
METHODS
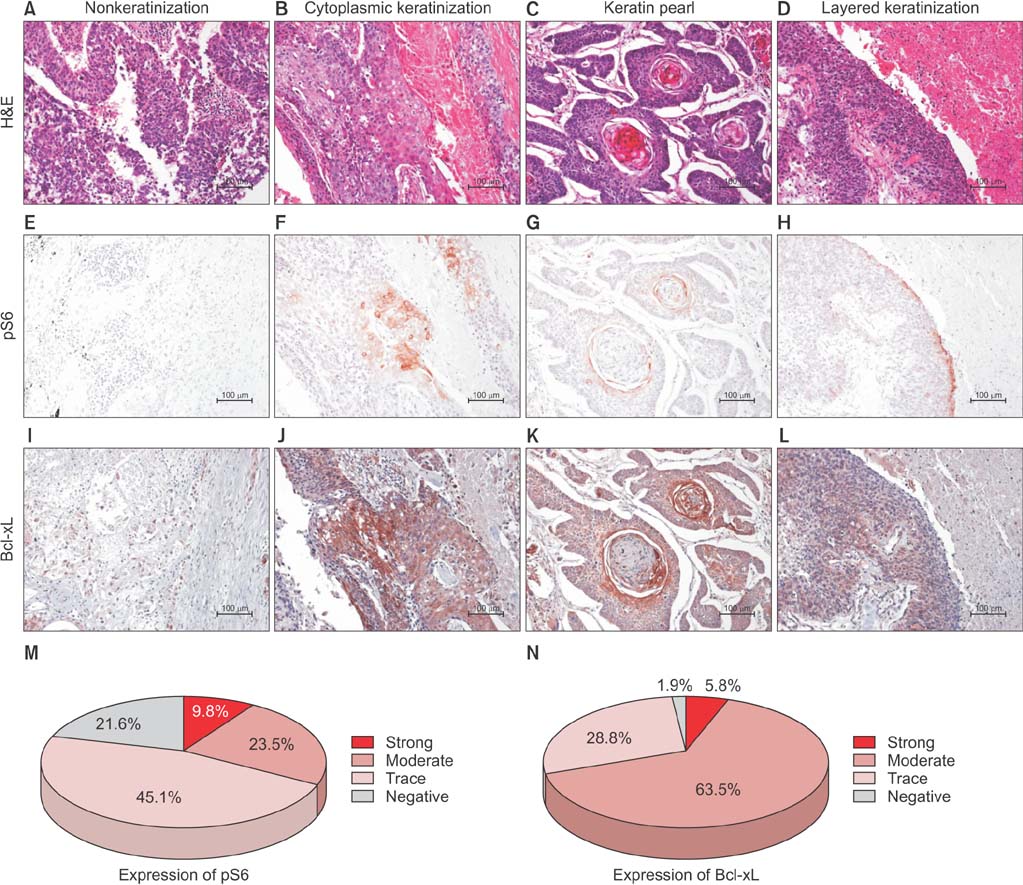
A total of 81 patients who underwent curative surgical resection of diagnosed lung SCC, were enrolled in this study. Attributes such as keratinization, tumor budding, single cell invasion, and nuclear size within the tumor, as well as immunohistochemistry of Bcl-xL and pS6 expressions, were evaluated.
RESULTS
The keratinizing and nonkeratinizing subtypes did not differ with respect to age, sex, TNM stage, and morphologic parameters such as nuclear diameter, tumor budding, and single cell invasion at the tumor edge. Most patients with the keratinizing subtype (98.0%) had a history of smoking, whereas the nonkeratinizing group had a relatively higher proportion of never-smokers relative to the keratinizing group (24.0% vs. 2.0%; p=0.008, chi-square test). Expression of pS6 (a surrogate marker of mammalian target of rapamycin complex 1 [mTORC1] signaling that regulates keratinocyte differentiation), and Bcl-xL (a key anti-apoptotic molecule that may inhibit keratinization), did not correlate significantly with the presence of keratinization. Patients with the keratinizing subtype had a significantly shorter overall survival (85.2 months vs. 135.7 months, p=0.010, log-rank test), and a multivariate analysis showed that keratinization was an independent, poor prognostic factor (hazard ratio, 2.389; 95% confidence interval, 1.090-5.233; p=0.030).
CONCLUSION
In lung SCC, keratinization is associated with a poor prognosis, and might be associated with smoking.
Keyword
MeSH Terms
Figure
Reference
-
1. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015; 10:1243–1260.2. Cardesa A, Nadal A. Carcinoma of the head and neck in the HPV era. Acta Dermatovenerol Alp Pannonica Adriat. 2011; 20:161–173.3. Yoshikawa H, Ehrhart EJ, Charles JB, Custis JT, LaRue SM. Assessment of predictive molecular variables in feline oral squamous cell carcinoma treated with stereotactic radiation therapy. Vet Comp Oncol. 2016; 14:39–57.4. Cooper T, Biron VL, Adam B, Klimowicz AC, Puttagunta L, Seikaly H. Association of keratinization with 5-year disease-specific survival in oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015; 141:250–256.5. Kadota K, Nitadori J, Woo KM, Sima CS, Finley DJ, Rusch VW, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol. 2014; 9:1126–1139.6. Ohbu M, Saegusa M, Okayasu I. Apoptosis and cellular proliferation in oesophageal squamous cell carcinomas: differences between keratinizing and nonkeratinizing types. Virchows Arch. 1995; 427:271–276.7. Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997; 3:614–620.8. Lu QL, Abel P, Foster CS, Lalani EN. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996; 27:102–110.9. Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One. 2014; 9:e103698.10. Wiedmann MW, Caca K. Molecularly targeted therapy for gastrointestinal cancer. Curr Cancer Drug Targets. 2005; 5:171–193.11. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002; 8:128–135.12. Zhu Q, Liang X, Dai J, Guan X. Prostaglandin transporter, SLCO2A1, mediates the invasion and apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 2015; 8:9175–9181.13. Jeong EH, Choi HS, Lee TG, Kim HR, Kim CH. Dual inhibition of PI3K/Akt/mTOR pathway and role of autophagy in non-small cell lung cancer cells. Tuberc Respir Dis (Seoul). 2012; 72:343–351.14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.15. Wenig BM, Cohen JM. General principles of management of head and neck cancer. In : Harrison LB, Sessions RB, Hong WK, editors. Head and neck cancer: a multidisciplinary approach. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2009. p. 3–50.16. Kim EY, Kim A, Kim SK, Kim HJ, Chang J, Ahn CM, et al. KRAS oncogene substitutions in Korean NSCLC patients: clinical implication and relationship with pAKT and RalGTPases expression. Lung Cancer. 2014; 85:299–305.17. Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Biophys Acta. 2013; 1833:3471–3480.18. Yoshida N, Egami H, Yamashita J, Takai E, Tamori Y, Fujino N, et al. Immunohistochemical expression of SKALP/elafin in squamous cell carcinoma of human lung. Oncol Rep. 2002; 9:495–501.19. Das DK, Chakraborty C, Sawaimoon S, Maiti AK, Chatterjee S. Automated identification of keratinization and keratin pearl area from in situ oral histological images. Tissue Cell. 2015; 47:349–358.20. Crissman JD, Pajak TF, Zarbo RJ, Marcial VA, Al-Sarraf M. Improved response and survival to combined cisplatin and radiation in non-keratinizing squamous cell carcinomas of the head and neck. An RTOG study of 114 advanced stage tumors. Cancer. 1987; 59:1391–1397.21. Cai C, Chernock RD, Pittman ME, El-Mofty SK, Thorstad WL, Lewis JS Jr. Keratinizing-type squamous cell carcinoma of the oropharynx: p16 overexpression is associated with positive high-risk HPV status and improved survival. Am J Surg Pathol. 2014; 38:809–815.22. Slebos RJ, Jehmlich N, Brown B, Yin Z, Chung CH, Yarbrough WG, et al. Proteomic analysis of oropharyngeal carcinomas reveals novel HPV-associated biological pathways. Int J Cancer. 2013; 132:568–579.23. Xiao D, Jia J, Shi Y, Fu C, Chen L, Jiang Y, et al. Opposed expression of IKKalpha: loss in keratinizing carcinomas and gain in non-keratinizing carcinomas. Oncotarget. 2015; 6:25499–25505.24. Michcik A, Cichorek M, Daca A, Chomik P, Wojcik S, Zawrocki A, et al. Tobacco smoking alters the number of oral epithelial cells with apoptotic features. Folia Histochem Cytobiol. 2014; 52:60–68.25. Ahmed HG, Ebnoof SO, Hussein MO, Gbreel AY. Oral epithelial atypical changes in apparently healthy oral mucosa exposed to smoking, alcohol, peppers and hot meals, using the AgNOR and Papanicolaou staining techniques. Diagn Cytopathol. 2010; 38:489–495.26. Orellana-Bustos AI, Espinoza-Santander IL, Franco-Martinez ME, Lobos-James-Freyre N, Ortega-Pinto AV. Evaluation of keratinization and AgNORs count in exfoliative cytology of normal oral mucosa from smokers and non-smokers. Med Oral. 2004; 9:197–203.27. Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000; 11:383–408.28. Xiang X, Zhao J, Xu G, Li Y, Zhang W. mTOR and the differentiation of mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai). 2011; 43:501–510.29. Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998; 16:395–419.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple primary lung cancer: Synchronous small cell lung carcinoma and squamous cell carcinoma
- Druggable Targets of Squamous Cell Lung Cancer
- Basaloid Squamous Cell Carcinoma of the Lung: Two Case Reports with CT Imaging Findings
- p40 Immunohistochemistry Is an Excellent Marker in Primary Lung Squamous Cell Carcinoma
- Metastatic Large Cell Neuroendocrine Carcinoma of the Lung Mimicking a Merkel Cell Carcinoma