Tuberc Respir Dis.
2017 Apr;80(2):105-112. 10.4046/trd.2017.80.2.105.
Spirometry and Bronchodilator Test
- Affiliations
-
- 1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Korea.
- 2Department of Allergy, Pulmonary and Critical Care Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 3Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Korea.
- 5Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 7Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. hs1017@ewha.ac.kr
- 8Division of Pulmonology and Critical Care, Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea.
- KMID: 2375980
- DOI: http://doi.org/10.4046/trd.2017.80.2.105
Abstract
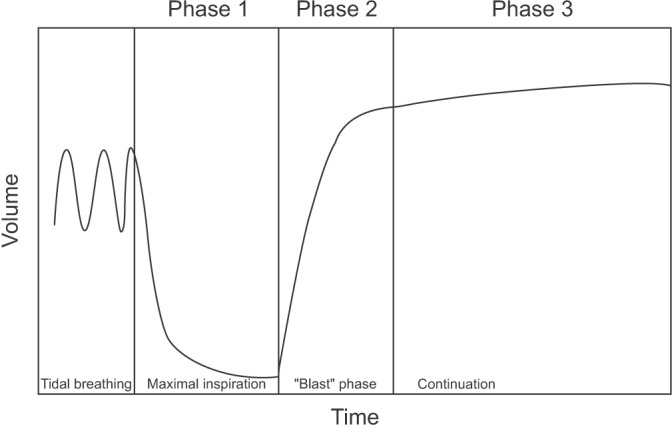
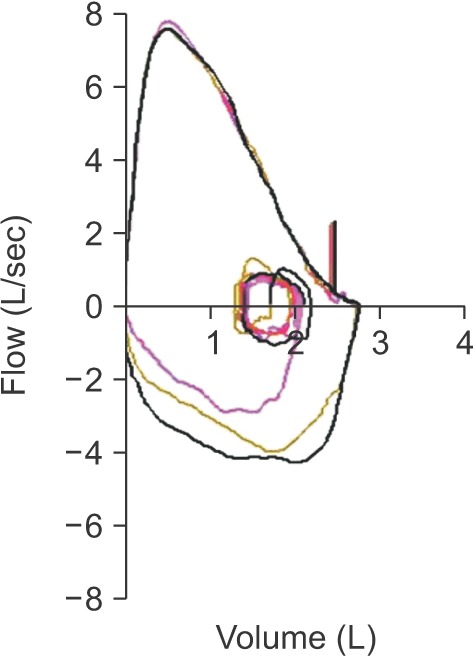
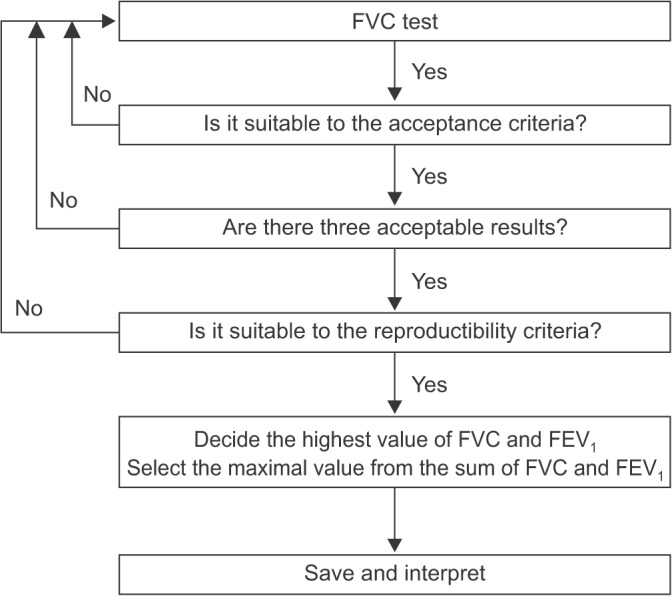
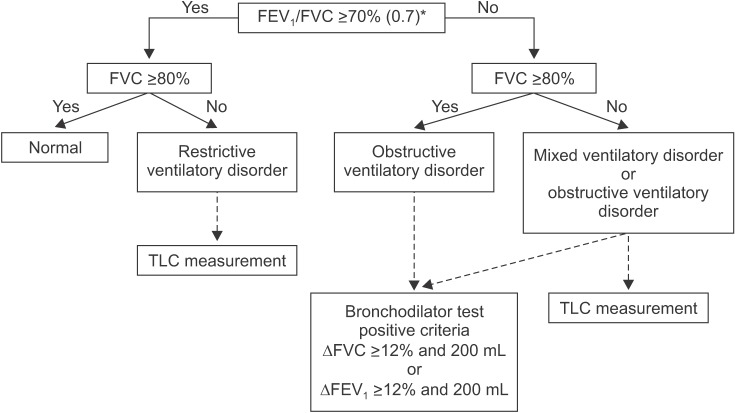
- Spirometry is a physiological test for assessing the functional aspect of the lungs using an objective indicator to measure the maximum amount of air that a patient can inhale and exhale. Acceptable spirometry testing needs to be conducted three times by an acceptable and reproducible method for determining forced vital capacity (FVC). Until the results of three tests meet the criteria of reproducibility, the test should be repeated up to eight times. Interpretation of spirometry should be clear, concise, and informative. Additionally, spirometry should guarantee optimal quality prior to the interpreting spirometry results. Our guideline adopts a fixed normal predictive value instead of the lower limit of normal as the reference value because fixed value is more convenient and also accepts FVC instead of vital capacity (VC) because measurement of VC using a spirometer is impossible. The bronchodilator test is a method for measuring the changes in lung capacity after inhaling a short-acting β-agonist that dilates the airway. When an obstructive ventilatory defect is observed, this test helps to diagnose and evaluate asthma and chronic obstructive pulmonary disease by measuring reversibility with the use of an inhaled bronchodilator. A positive response to a bronchodilator is generally defined as an increase of ≥12% and ≥200 mL as an absolute value compared with a baseline in either forced expiratory volume at 1 second or FVC.
Keyword
MeSH Terms
Figure
Reference
-
1. The BTS COPD Consortium. Spirometry in practice. A practical guide to using spirometry in primary care. 2nd ed. London: The BTS COPD Consortium;2005.2. The Korean Academy of Tuberculosis and Respiratory Diseases. 2016 Guideline of pulmonary function test [Internet]. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases;2016. cited 2016 Nov 1. Available from: http://www.lungkorea.org/bbs/index.html?code=guide&category=&gubun=&page=1&number=3487&mode=view&keyfield=&key=.3. Gold WM, Koth LL. Chapter 25. Pulmonary function testing. In : Broaddus VC, Mason RJ, Ernst JD, King TE, Lazarus SC, Murray JF, editors. Murray and Nadel's textbook of respiratory medicine. 6th ed. Philadelphia: Saunders/Elsevier;2015. p. 407–435.e18.4. Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005; 26:153–161. PMID: 15994402.5. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338. PMID: 16055882.6. Moore VC. Spirometry: step by step. Breathe. 2012; 8:232–240.
Article7. Centers for Disease Control and Prevention. The Fifth Korea National Health and Nutrition Examination Survey (2010-2012) Manual [Internet]. Cheongju: Centers for Disease Control and Prevention;2012. cited 2016 Nov 1. Available from: http://cdc.go.kr/CDC/contents/CdcKrContentView.jsp?cid=60948&menuIds=HOME001-MNU1130-MNU1639-MNU1749-MNU1760.8. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): respiratory health spirometry procedures manual [Internet]. Atlanta: Centers for Disease Control and Prevention;2011. cited 2016 Nov 1. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Spirometry_Procedures_Manual.pdf.9. Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971; 103:57–67. PMID: 5540840.
Article10. Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005; 58:230–242.
Article11. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007; 176:532–555. PMID: 17507545.12. Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008; 63:1046–1051. PMID: 18786983.
Article13. Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008; 63:1040–1045. PMID: 18492741.14. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26:948–968. PMID: 16264058.15. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet]. London: National Institute for Health and Care Excellence;2004. cited 2016 Nov 1. Available from: http://www.nice.org.uk/guidance/cg12.16. Peces-Barba G, Barbera JA, Agusti A, Casanova C, Casas A, Izquierdo JL, et al. Diagnosis and management of chronic obstructive pulmonary disease: joint guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Latin American Thoracic Society (ALAT). Arch Bronconeumol. 2008; 44:271–281. PMID: 18448019.17. American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991; 144:1202–1218. PMID: 1952453.18. Ministry of Health and Welfare. The criteria for decision of pulmonary disability-grading: notification No. 2015-188 [Internet]. Sejong: Ministry of Health and Welfare;2015. cited 2016 Nov 1. Available from: http://www.mohw.go.kr/front_new/jb/sjb0406ls.jsp?PAR_MENU_ID=03&MENU_ID=030406&page=30.19. National Center for Health Statistics. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): respiratory health bronchodilator procedure manual [Internet]. Atlanta: Centers for Disease Control and Prevention;2008. cited 2016 Nov 1. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/Bronchodilator.pdf.20. van Schalkwyk EM, Schultz C, Joubert JR, White NW. South African Thoracic Society Standards of Spirometry Committee. Guideline for office spirometry in adults, 2004. S Afr Med J. 2004; 94(7 Pt 2):576–587. PMID: 15283308.21. Global Initiative for Asthma. Diagnosis of diseases of chronic airflow limitation: 2015 asthma, COPD and asthma-COPD overlap syndrome (ACOS) [Internet]. Global Initiative for Asthma;2015. cited 2016 Oct 12. Available from: http://ginasthma.org/asthma-copd-and-asthma-copd-overlap-syndrome-acos.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severity Staging of Chronic Obstructive Pulmonary Disease: Differences in Pre- and Post-Bronchodilator Spirometry
- Role of Bronchodilator Reversibility Testing in Differentiating Asthma From COPD
- Prediction of Brobchodilator Response by Using FEF25~75% in Adult Patient with a Normal Spirometry Result
- The Relation Between Bronchodilator Response, Airway Hyperresponsiveness and Serum Eosinophil Cationic Protein (ECP) Level in Moderate to Severe Asthmatics
- Relationship between bronchiectasis with wheeze and asthma