J Cardiovasc Ultrasound.
2017 Mar;25(1):20-27. 10.4250/jcu.2017.25.1.20.
Role of Quantitative Wall Motion Analysis in Patients with Acute Chest Pain at Emergency Department
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Sejong General Hospital, Bucheon, Korea.
- 2Division of Cardiology, Department of internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. imnash70@snu.ac.kr
- 3Institute on Aging, Seoul National University, Seoul, Korea.
- KMID: 2375293
- DOI: http://doi.org/10.4250/jcu.2017.25.1.20
Abstract
- BACKGROUND
Evaluation of acute chest pain in emergency department (ED), using limited resource and time, is still very difficult despite recent development of many diagnostic tools. In this study, we tried to determine the applicability of new semi-automated cardiac function analysis tool, velocity vector imaging (VVI), in the evaluation of the patients with acute chest pain in ED.
METHODS
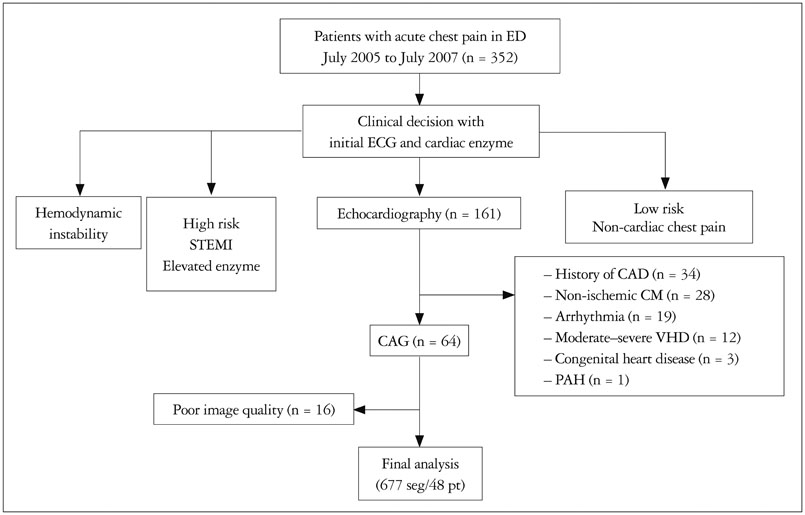
We prospectively enrolled 48 patients, who visited ED with acute chest pain, and store images to analyze VVI from July 2005 to July 2007.
RESULTS
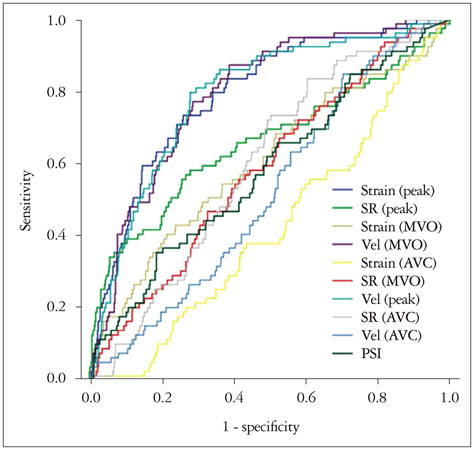
In 677 of 768 segments (88%), the analysis by VVI was feasible among 48 patients. Peak systolic radial velocity (V(peak)) and strain significantly decreased according to visual regional wall motion abnormality (V(peak), 3.50 ± 1.34 cm/s for normal vs. 3.46 ± 1.52 cm/s for hypokinesia, 2.51 ± 1.26 for akinesia, p < 0.01; peak systolic radial strain -31.74 ± 9.15% fornormal, -24.33 ± 6.28% for hypokinesia, -20.30 ± 7.78% for akinesia, p < 0.01). However, the velocity vectors at the time of mitral valve opening (MVO) were directed outward in the visually normal myocardium, inward velocity vectors were revealed in the visually akinetic area (V(MVO), -0.85 ± 1.65 cm/s for normal vs. 0.10 ± 1.46 cm/s for akinesia, p < 0.001). At coronary angiography, V(MVO) clearly increased in the ischemic area (V(MVO), -0.88+1.56 cm/s for normal vs. 0.70 + 2.04 cm/s for ischemic area, p < 0.01).
CONCLUSION
Regional wall motion assessment using VVI showed could be used to detect significant ischemia in the patient with acute chest pain at ED.
MeSH Terms
Figure
Cited by 2 articles
-
Ergonovine Provocation Echocardiography for Detection and Prognostication in Patients with Vasospastic Angina
Jae-Hyeong Park
Korean Circ J. 2018;48(10):917-919. doi: 10.4070/kcj.2018.0139.Two-dimensional Echocardiographic Assessment of Myocardial Strain: Important Echocardiographic Parameter Readily Useful in Clinical Field
Jae-Hyeong Park
Korean Circ J. 2019;49(10):908-931. doi: 10.4070/kcj.2019.0200.
Reference
-
1. Selker HP, Beshansky JR, Griffith JL, Aufderheide TP, Ballin DS, Bernard SA, Crespo SG, Feldman JA, Fish SS, Gibler WB, Kiez DA, McNutt RA, Moulton AW, Ornato JP, Podrid PJ, Pope JH, Salem DN, Sayre MR, Woolard RH. Use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist with triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia. A multicenter, controlled clinical trial. Ann Intern Med. 1998; 129:845–855.2. Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, Griffith JL, Selker HP. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000; 342:1163–1170.3. Than M, Cullen L, Reid CM, Lim SH, Aldous S, Ardagh MW, Peacock WF, Parsonage WA, Ho HF, Ko HF, Kasliwal RR, Bansal M, Soerianata S, Hu D, Ding R, Hua Q, Seok-Min K, Sritara P, Sae-Lee R, Chiu TF, Tsai KC, Chu FY, Chen WK, Chang WH, Flaws DF, George PM, Richards AM. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011; 377:1077–1084.4. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.5. Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003; 107:2120–2126.6. Langeland S, D'hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, Sutherland GR. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005; 112:2157–2162.7. Cannesson M, Tanabe M, Suffoletto MS, Schwartzman D, Gorcsan J 3rd. Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol. 2006; 98:949–953.8. Kim DH, Kim HK, Kim MK, Chang SA, Kim YJ, Kim MA, Sohn DW, Oh BH, Park YB. Velocity vector imaging in the measurement of left ventricular twist mechanics: head-to-head one way comparison between speckle tracking echocardiography and velocity vector imaging. J Am Soc Echocardiogr. 2009; 22:1344–1352.9. Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987; 59:23C–30C.10. Sasaki H, Charuzi Y, Beeder C, Sugiki Y, Lew AS. Utility of echocardiography for the early assessment of patients with nondiagnostic chest pain. Am Heart J. 1986; 112:494–497.11. Grenne B, Eek C, Sjøli B, Dahlslett T, Uchto M, Hol PK, Skulstad H, Smiseth OA, Edvardsen T, Brunvand H. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: outcome and early identification by strain echocardiography. Heart. 2010; 96:1550–1556.12. Kukulski T, Jamal F, Herbots L, D'hooge J, Bijnens B, Hatle L, De Scheerder I, Sutherland GR. Identification of acutely ischemic myocardium using ultrasonic strain measurements. A clinical study in patients undergoing coronary angioplasty. J Am Coll Cardiol. 2003; 41:810–819.13. Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002; 106:50–56.14. Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000; 102:1158–1164.15. Asanuma T, Masuda K, Taniguchi A, Uranishi A, Ishikura F, Beppu S. Spatial extent of postsystolic thickening during myocardial ischemia: evaluation by velocity vector imaging. J Echocardiogr. 2006; 4:84–85.16. Claus P, Weidemann F, Dommke C, Bito V, Heinzel FR, D'hooge J, Sipido KR, Sutherland GR, Bijnens B. Mechanisms of postsystolic thickening in ischemic myocardium: mathematical modelling and comparison with experimental ischemic substrates. Ultrasound Med Biol. 2007; 33:1963–1970.17. Masuda K, Asanuma T, Taniguchi A, Uranishi A, Ishikura F, Beppu S. Assessment of dyssynchronous wall motion during acute myocardial ischemia using velocity vector imaging. JACC Cardiovasc Imaging. 2008; 1:210–220.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Echocardiography for Evaluation of the Acute Chest Pain Patients with Nonspecific ECG Findings
- Role of Echocardiography in the Emergency Department
- Quantitative Two-Dimensional Echocardiographic Analysis of Left Ventricular Wall Motion in Patients with Acute Myocardial Infarction
- Computerized Quantitative Analysis of Left Ventricular Wall Motion by 2-Dimensional Echocardiography
- The Clinical Feature and Pressure Threshold in a Chest Wall Syndrome