Lab Anim Res.
2017 Mar;33(1):32-39. 10.5625/lar.2017.33.1.32.
Immunomodulatory effects of ethanol extract of germinated ice plant (Mesembryanthemum crystallinum)
- Affiliations
-
- 1Laboratory Animal Medicine, College of Veterinary Medicine and BK 21 PLUS Project Team, Chonnam National University, Gwangju, Korea. jonpark@jnu.ac.kr
- 2Institute of Biotechnology, Bioresource Inc., Cheomdan venture, Gwangju, Korea.
- 3Department of Rheumatology, Chonnam National University Medical School, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 2375270
- DOI: http://doi.org/10.5625/lar.2017.33.1.32
Abstract
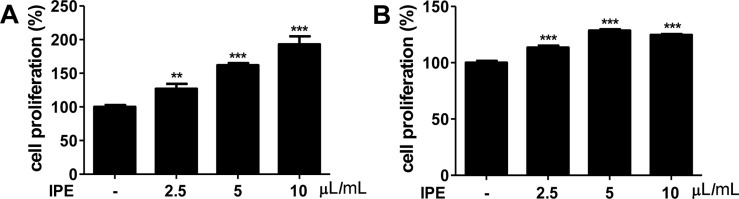
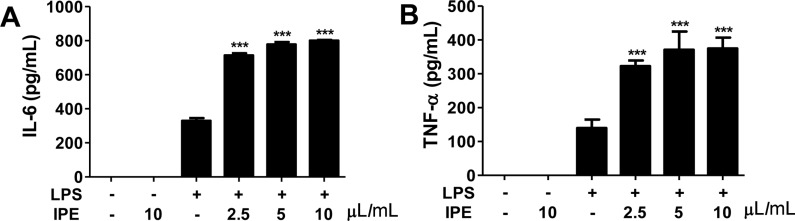
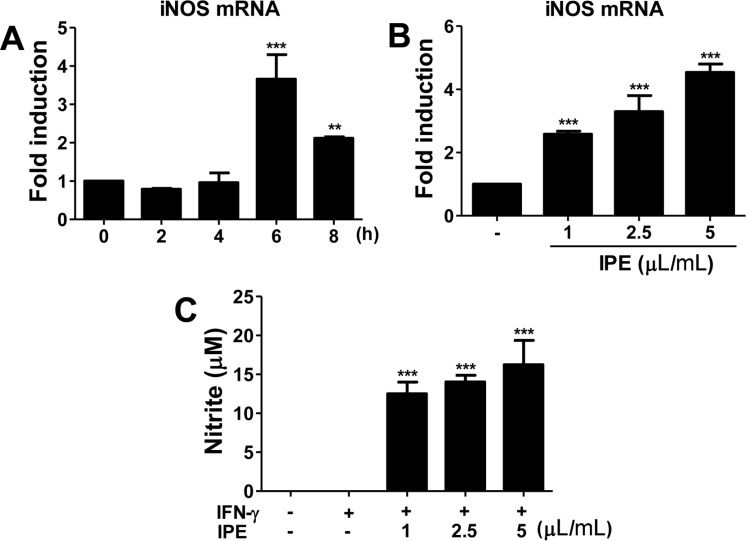
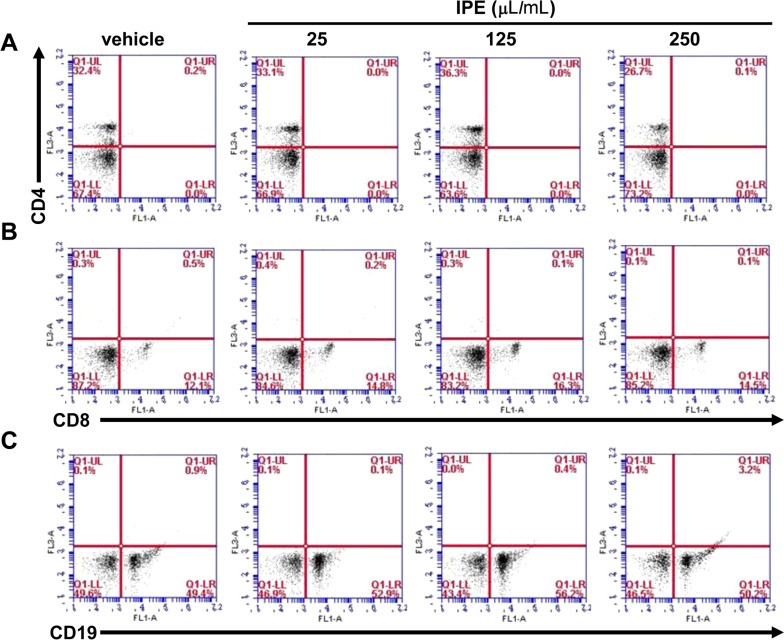
- The purpose of this study was to investigate the immunomodulatory activity of ice plant (Mesembryanthemum crystallinum) extract (IPE) in vitro and in vivo. Raji (a human B cell line) and Jurkat (a human T cell line) cells were treated with various doses of IPE and cell proliferation was measured by WST assay. Results showed that IPE promoted the proliferation of both Raji and Jurkat cells in a dose-dependent manner. IPE also enhanced IL-6 and TNF-α production in macrophages in the presence of lipopolysaccharide (LPS), although IPE alone did not induce cytokine production. Moreover, IPE treatment upregulated iNOS gene expression in macrophages in a time- and dose-dependent manner and led to the production of nitric oxide in macrophages in the presence of IFNγ. In vivo studies revealed that oral administration of IPE for 2 weeks increased the differentiation of CD4+, CD8+, and CD19+ cells in splenocytes. These findings suggested that IPE has immunomodulatory effects and could be developed as an immunomodulatory supplement.
MeSH Terms
Figure
Reference
-
1. Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007; 219:187–203. PMID: 17850490.
Article2. Wang ML, et al. Immunomodulatory activities of Gelidium amansii gel extracts on murine RAW 264.7 macrophages. J Food Drug Anal. 2013; 21(4):397–403.
Article3. Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993; 177(2):511–516. PMID: 8426119.
Article4. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002; 4(Suppl 3):S233–S242. PMID: 12110143.5. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2(10):907–916. PMID: 11577346.
Article6. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012; 54(Suppl):S29–S37. PMID: 22178471.
Article7. Miyazaki Y, Kusano S, Doi H, Aki O. Effects on immune response of antidiabetic ingredients from white-skinned sweet potato (Ipomoea batatas L.). Nutrition. 2005; 21(3):358–362. PMID: 15797679.
Article8. Lin BF, Chiang BL, Lin JY. Amaranthus spinosus water extract directly stimulates proliferation of B lymphocytes in vitro. Int Immunopharmacol. 2005; 5(4):711–722. PMID: 15710340.9. Chen X, Nie W, Yu G, Li Y, Hu Y, Lu J, Jin L. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem Toxicol. 2012; 50(3-4):695–700. PMID: 22120506.
Article10. Thakur M, Connellan P, Deseo MA, Morris C, Praznik W, Loeppert R, Dixit VK. Characterization and in vitro immunomodulatory screening of fructo-oligosaccharides of Asparagus racemosus Willd. Int J Biol Macromol. 2012; 50(1):77–81. PMID: 22001723.11. Tsukagoshi H, Suzuki T, Nishikawa K, Agarie S, Ishiguro S, Higashiyama T. RNA-seq analysis of the response of the halophyte, Mesembryanthemum crystallinum (ice plant) to high salinity. PLoS One. 2015; 10(2):e0118339. PMID: 25706745.
Article12. AMARASINGHE V, WATSON L. Comparative ultrastructure of microhairs in grasses. Bot J Linn Soc. 1988; 98(4):303–319.
Article13. Vogt T, Ibdah M, Schmidt J, Wray V, Nimtz M, Strack D. Light-induced betacyanin and flavonol accumulation in bladder cells of Mesembryanthemum crystallinum. Phytochemistry. 1999; 52(4):583–592. PMID: 10570827.
Article14. Deters AM, Meyer U, Stintzing FC. Time-dependent bioactivity of preparations from cactus pear (Opuntia ficus indica) and ice plant (Mesembryanthemum crystallinum) on human skin fibroblasts and keratinocytes. J Ethnopharmacol. 2012; 142(2):438–444. PMID: 22713931.
Article15. Drira R, Matsumoto T, Agawa M, Sakamoto K. Ice Plant (Mesembryanthemum crystallinum) Extract Promotes Lipolysis in Mouse 3T3-L1 Adipocytes Through Extracellular Signal-Regulated Kinase Activation. J Med Food. 2016; 19(3):274–280. PMID: 26390196.
Article16. Sivakumar S, Subramanian SP. Pancreatic tissue protective nature of D-Pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur J Pharmacol. 2009; 622(1-3):65–70. PMID: 19765586.
Article17. Lee BH, Lee CC, Wu SC. Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J Sci Food Agric. 2014; 94(11):2266–2273. PMID: 24374864.
Article18. Lin TH, Tan TW, Tsai TH, Chen CC, Hsieh TF, Lee SS, Liu HH, Chen WC, Tang CH. D-pinitol inhibits prostate cancer metastasis through inhibition of αVβ3 integrin by modulating FAK, c-Src and NF-κB pathways. Int J Mol Sci. 2013; 14(5):9790–9802. PMID: 23698767.19. Singh RK, Pandey BL, Tripathi M, Pandey VB. Anti-inflammatory effect of (+)-pinitol. Fitoterapia. 2001; 72(2):168–170. PMID: 11223227.
Article20. Celada A, Gray PW, Rinderknecht E, Schreiber RD. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984; 160(1):55–74. PMID: 6330272.
Article21. Kim H, Lee HS, Chang KT, Ko TH, Baek KJ, Kwon NS. Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-kappa B. J Immunol. 1995; 154(9):4741–4748. PMID: 7536778.22. Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000; 83(2):167–176. PMID: 10743496.23. Ghonime M, Emara M, Shawky R, Soliman H, El-Domany R, Abdelaziz A. Immunomodulation of RAW 264.7 murine macrophage functions and antioxidant activities of 11 plant extracts. Immunol Invest. 2015; 44(3):237–252. PMID: 25564700.
Article24. Glauser MP. The inflammatory cytokines. New developments in the pathophysiology and treatment of septic shock. Drugs. 1996; 52(Suppl 2):9–17.25. Hirohashi N, Morrison DC. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect Immun. 1996; 64(3):1011–1015. PMID: 8641750.26. Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol. 2006; 2(11):602–610. PMID: 17075599.
Article27. Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004; 199(5):731–736. PMID: 14981113.28. Kehrl JH, Miller A, Fauci AS. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987; 166(3):786–791. PMID: 3040886.
Article29. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441(7090):235–238. PMID: 16648838.
Article30. Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995; 86(4):1243–1254. PMID: 7632928.
Article31. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997; 15:323–350. PMID: 9143691.
Article32. Suhkneung P, et al. Immunomodulatory Effects of Cimicifugae Rhizoma Extracts in Macrophages. Prev Nutr Food Sci. 2006; 11(4):268–272.33. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4 T cells in immunity to viruses. Nat Rev Immunol. 2012; 12(2):136–148. PMID: 22266691.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant and growth inhibitory activities of Mesembryanthemum crystallinum L. in HCT116 human colon cancer cells
- Immunomodulatory Effects of Callophyllis japonica Ethanol Extract on Dendritic Cells
- Effect of medicinal plant extract for hangover relief
- Immunomodulatory effects mixed with Weissella cibaria JW15 and Black soybean (Glycine max (L.) Merr.) Extract
- Cell Beath Induced by Ethanol : Prevention of Cell Death by the bcl-2 Proto-Oncogene