Lab Anim Res.
2017 Mar;33(1):15-23. 10.5625/lar.2017.33.1.15.
The effects of pentoxifylline adminstration on fracture healing in a postmenopausal osteoporotic rat model
- Affiliations
-
- 1Basic Sciences Department, Paramedical School, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Department of Anatomy, School of medicine, Iran University of Medical Sciences, Tehran, Iran.
- 3Department of Anatomical sciences and Biology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 4Cellular and Molecular Biology Research Centre, Department of Anatomical Sciences and Biology, Medical School, Shahid Beheshti University of Medical Sciences, Tehran, Iran. bayat_m@yahoo.com mohbayat@sbmu.ac.ir
- KMID: 2375268
- DOI: http://doi.org/10.5625/lar.2017.33.1.15
Abstract
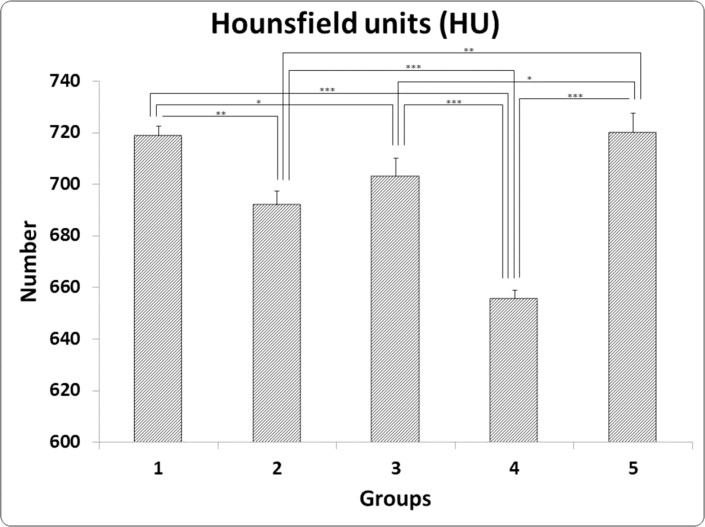
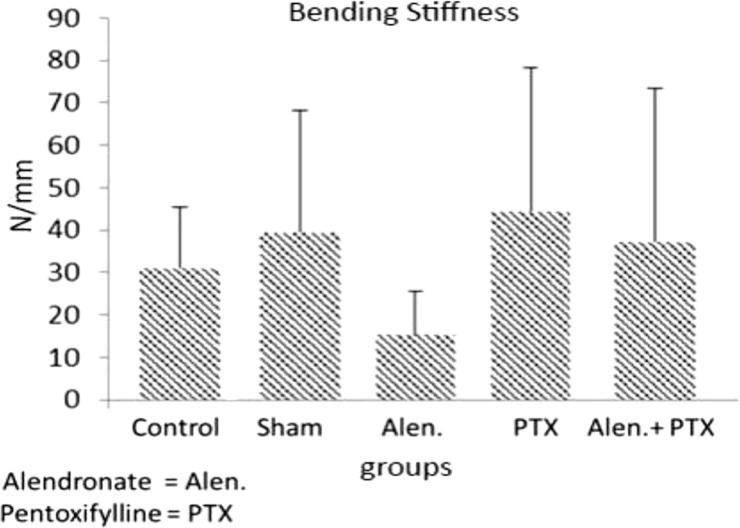
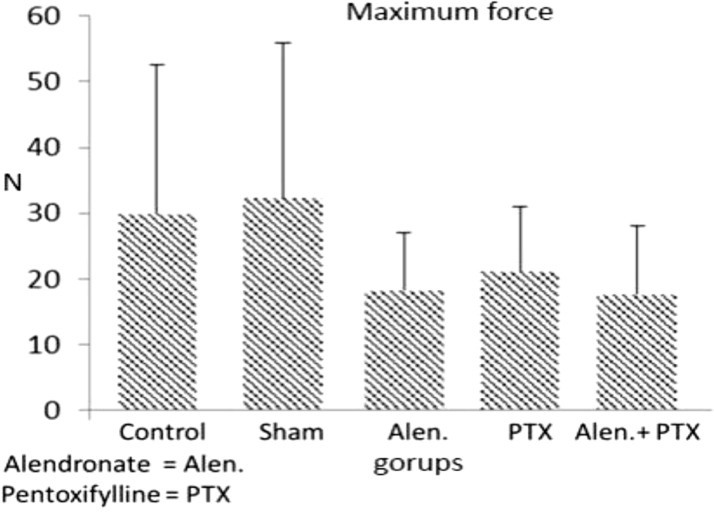
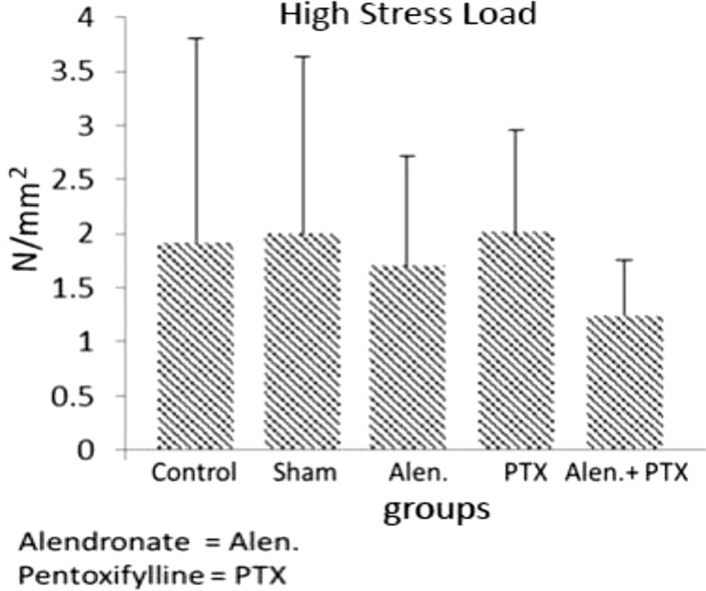
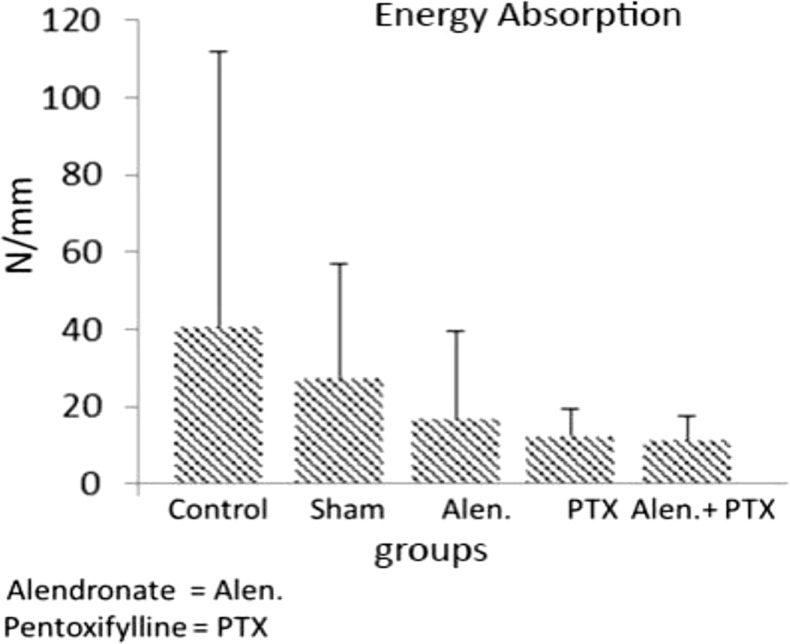
- Previous studies report positive effects of pentoxifylline (PTX) alone or in combination with other drugs on some pathologic bone diseases as well as an ability to accelerate osteogensis and fracture healing in both animal models and human patients. The aim of this present study was to evaluate the effects of PTX administration on Hounsfield unit and bone strength at catabolic response (bone resorbing) of a fracture in an experimental rat model of ovariectomy induced osteoporosis (OVX-D). Thirty adult female rats were divided into groups as follows: 1 (OVX, control, no treatment); 2 (OVX, sham: daily distilled water); 3 (OVX, daily alendronate: 3 mg/kg); 4 (OVX, twice daily 100 mg/kg PTX) and 5 (OVX, PTX+alenderonate). OVX was induced by bilateral ovariectomy in all rats. A complete standardized osteotomy of the right femur was made after 3.5 months. PTX and alendronate treatments were performed for eight weeks. Then, rats were euthanized and had its right femur subjected to computerized tomography scanning for measuring Hounsfield unit; eventually, the samples were sent for a three point bending test for evaluation of the bone strength. Administration of PTX with 200 mg/kg and alendronate alone and in combination showed no significant alteration in Hounsfield unit and biomechanical properties of repairing callus of the complete osteotomy compared with the control group. Results showed increased bending stiffness and stress high load mean values of repairing complete osteotomy in PTX-treated rats compared to the control OVX-D.
MeSH Terms
Figure
Reference
-
1. Ozgediz D, Chu K, Ford N, Dubowitz G, Bedada AG, Azzie G, Gerstle JT, Riviello R. Surgery in global health delivery. Mt Sinai J Med. 2011; 78(3):327–341. PMID: 21598260.
Article2. Mock C, Cherian MN. The global burden of musculoskeletal injuries: challenges and solutions. Clin Orthop Relat Res. 2008; 466(10):2306–2316. PMID: 18679760.
Article3. Bandela V, Munagapati B, Karnati RK, Venkata GR, Nidudhur SR. Osteoporosis: Its Prosthodontic Considerations-A Review. J Clin Diagn Res. 2015; 9(12):ZE01–ZE04.4. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006; 17(12):1726–1733. PMID: 16983459.
Article5. Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997; 12(1):24–35. PMID: 9240722.
Article6. Tajeu GS, Delzell E, Smith W, Arora T, Curtis JR, Saag KG, Morrisey MA, Yun H, Kilgore ML. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci. 2014; 69(3):346–353. PMID: 23873945.
Article7. Pothiwala P, Evans EM, Chapman-Novakofski KM. Ethnic variation in risk for osteoporosis among women: a review of biological and behavioral factors. J Womens Health (Larchmt). 2006; 15(6):709–719. PMID: 16910903.
Article8. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21(2):115–137. PMID: 10782361.
Article9. Ralston SH. Analysis of gene expression in human bone biopsies by polymerase chain reaction: evidence for enhanced cytokine expression in postmenopausal osteoporosis. J Bone Miner Res. 1994; 9(6):883–890. PMID: 8079663.
Article10. Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, Wakabayashi S, Takaoka K. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone. 2000; 27(6):811–817. PMID: 11113392.
Article11. Ahlström M, Lamberg-Allardt C. Rapid protein kinase A--mediated activation of cyclic AMP-phosphodiesterase by parathyroid hormone in UMR-106 osteoblast-like cells. J Bone Miner Res. 1997; 12(2):172–178. PMID: 9041048.
Article12. Civitelli R, Bacskai BJ, Mahaut-Smith MP, Adams SR, Avioli LV, Tsien RY. Single-cell analysis of cyclic AMP response to parathyroid hormone in osteoblastic cells. J Bone Miner Res. 1994; 9(9):1407–1417. PMID: 7817824.
Article13. Wronski TJ, Yen CF, Qi H, Dann LM. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993; 132(2):823–831. PMID: 8425497.
Article14. Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 1990; 87(2):533–537. PMID: 2153956.
Article15. Marchmont RJ, Ayad SR, Houslay MD. Purification and properties of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981; 195(3):645–652. PMID: 6274308.
Article16. Bayat M, Amini A, Rezaie F, Bayat S. Patents of Pentoxifylline Administration on some diseases and chronic wounds. Recent Pat Regen Med. 2014; 4(2):137–143.
Article17. Vashghani Farahani MM, Masteri Farahani R, Mostafavinia A, Abbasian MR, Pouriran R, Noruzian M, Ghoreishi SK, Aryan A, Bayat M. Effect of Pentoxifylline Administration on an Experimental Rat Model of Femur Fracture Healing With Intramedullary Fixation. Iran Red Crescent Med J. 2015; 17(12):e29513. PMID: 26756019.
Article18. Çakmak G, Þahin MÞ, Özdemİ BH, Karadenİ E. Effect of pentoxifylline on healing of segmental bone defects and angiogenesis. Acta Orthop Traumatol Turc. 2015; 49(6):676–682. PMID: 26511696.19. Atalay Y, Gunes N, Guner MD, Akpolat V, Celik MS, Guner R. Pentoxifylline and electromagnetic field improved bone fracture healing in rats. Drug Des Devel Ther. 2015; 9:5195–5201.
Article20. Bohn JC, Schussel JL, Stramandinoli-Zanicotti RT, Sassi LM. Tissue repair in osteoradionecrosis using pentoxifylline and tocopherol--report of three cases. Oral Maxillofac Surg. 2016; 20(1):97–101. PMID: 26251132.
Article21. Erken HY, Burc H, Aydogan M. The Effect of Pentoxifylline on Spinal Fusion: An Experimental Study in Rabbits. Spine (Phila Pa 1976). 2014; 39(11):27.22. Labib GS, Farid RM. Osteogenic effect of locally applied Pentoxyfilline gel: in vitro and in vivo evaluations. Drug Deliv. 2015; 22(8):1094–1102. PMID: 24555662.23. Queiroz-Junior CM, Bessoni RL, Costa VV, Souza DG, Teixeira MM, Silva TA. Preventive and therapeutic anti-TNF-α therapy with pentoxifylline decreases arthritis and the associated periodontal co-morbidity in mice. Life Sci. 2013; 93(9-11):423–428. PMID: 23911669.
Article24. Aydin K, Sahin V, Gürsu S, Mercan AS, Demir B, Yildirim T. Effect of pentoxifylline on fracture healing: an experimental study. Eklem Hastalik Cerrahisi. 2011; 22(3):160–165. PMID: 22085352.25. Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009; 13(3):253–262. PMID: 18554967.
Article26. Horiuchi H, Saito N, Kinoshita T, Wakabayashi S, Tsutsumimoto T, Otsuru S, Takaoka K. Enhancement of recombinant human bone morphogenetic protein-2 (rhBMP-2)-induced new bone formation by concurrent treatment with parathyroid hormone and a phosphodiesterase inhibitor, pentoxifylline. J Bone Miner Metab. 2004; 22(4):329–334. PMID: 15221490.
Article27. Beþe NS, Ozgüroğ M, Kamberoğ K, Karahasanoglu T, Ober A. Pentoxifylline in the treatment of radiation-related pelvic insufficiency fractures of bone. Radiat Med. 2003; 21(5):223–227. PMID: 14632299.28. Robin JC, Ambrus JL. Study of antiosteoporotic agents in tissue culture. J Med. 1984; 15(4):319–322. PMID: 6098626.29. Robin JC, Ambrus JL. Studies on osteoporoses. XI. Effects of a methylxanthine derivative. A preliminary report. J Med. 1983; 14(2):137–145. PMID: 6310016.30. Magremanne M, Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg. 2014; 72(2):334–337. PMID: 23891014.
Article31. Takami M, Cho ES, Lee SY, Kamijo R, Yim M. Phosphodiesterase inhibitors stimulate osteoclast formation via TRANCE/RANKL expression in osteoblasts: possible involvement of ERK and p38 MAPK pathways. FEBS Lett. 2005; 579(3):832–838. PMID: 15670856.
Article32. Hosny KM. Alendronate sodium as enteric coated solid lipid nanoparticles; preparation, optimization, and in vivo evaluation to enhance its oral bioavailability. PLoS One. 2016; 11(5):e0154926. PMID: 27148747.33. Imai K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: review of a novel, easy to swallow formulation. Clin Interv Aging. 2013; 8:681–688. PMID: 23766643.
Article34. Mostafavinia A, Ahadi R, Abdollahifar MA, Ghorishi SK, Jalalifirouzkouhi A, Bayat M. Evaluation of the Effects of Low-Level Laser Therapy on Biomechanical Properties and Hounsfield Unit of Partial Osteotomy Healing in an Experimental Rat Model of Type I Diabetes and Osteoporosis. under publication. DOI: 10.1089/pho.2016.4191.35. Lee SW, Jeon TJ, Biswal S. Fracture Healing Effects of Locally-Administered Adipose Tissue-Derived Cells. Yonsei Med J. 2015; 56(4):1106–1113. PMID: 26069136.
Article36. Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br. 2007; 89(4):425–433. PMID: 17463107.
Article37. Caro AC, Tucker JJ, Yannascoli SM, Dunkman AA, Thomas SJ, Soslowsky LJ. Efficacy of various analgesics on shoulder function and rotator cuff tendon-to-bone healing in a rat (Rattus norvegicus) model. J Am Assoc Lab Anim Sci. 2014; 53(2):185–192. PMID: 24602546.38. Freidouni M, Nejati H, Salimi M, Bayat M, Amini A, Noruzian M, Asgharie MA, Rezaian M. Evaluating glucocorticoid administration on biomechanical properties of rats' tibial diaphysis. Iran Red Crescent Med J. 2015; 17(3):e19389. PMID: 26019900.
Article39. Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA. Effects of 830-nm laser, used in two doses, on biomechanical properties of osteopenic rat femora. Photomed Laser Surg. 2006; 24(2):202–206. PMID: 16706700.
Article40. Ibrahim N, Mohamed N, Shuid AN. Update on statins: hope for osteoporotic fracture healing treatment. Curr Drug Targets. 2013; 14(13):1524–1532. PMID: 23876090.
Article41. Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol. 2011; 7(11):631–638. PMID: 21931340.
Article42. Bayat M, Chelcheraghi F, Piryaei A, Rakhshan M, Mohseniefar Z, Rezaie F, Bayat M, Shemshadi H, Sadeghi Y. The effect of 30-day pretreatment with pentoxifylline on the survival of a random skin flap in the rat: an ultrastructural and biomechanical evaluation. Med Sci Monit. 2006; 12(6):BR201–BR207. PMID: 16733477.43. Velaei K, Bayat M, Torkman G, Rezaie F, Amini A, Noruzian M, Tavassol A, Bayat M. Evaluating the effects of pentoxifylline administration on experimental pressure sores in rats by biomechanical examinations. Lab Anim Res. 2012; 28(3):209–215. PMID: 23091522.
Article44. Babaei S, Bayat M, Nouruzian M, Bayat M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013; 700(1-3):165–172. PMID: 23220163.
Article45. Babaei S, Bayat M. Pentoxifylline Accelerates Wound Healing Process by Modulating Gene Expression of MMP-1, MMP-3, and TIMP-1 in Normoglycemic Rats. J Invest Surg. 2015; 28(4):196–201. PMID: 26087281.
Article46. Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med. 1998; 22(2):97–102. PMID: 9484702.
Article47. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003; 14(Suppl 3):S13–S18. PMID: 12730800.
Article48. Ammann P. [Determining factors of bone mechanical resistance]. Therapie. 2003; 58(5):403–407. PMID: 14682187.49. Wakabayashi S, Tsutsumimoto T, Kawasaki S, Kinoshita T, Horiuchi H, Takaoka K. Involvement of phosphodiesterase isozymes in osteoblastic differentiation. J Bone Miner Res. 2002; 17(2):249–256. PMID: 11811555.
Article50. Rawadi G, Ferrer C, Spinella-Jaegle S, Roman-Roman S, Bouali Y, Baron R. 1-(5-oxohexyl)-3,7-Dimethylxanthine, a phosphodiesterase inhibitor, activates MAPK cascades and promotes osteoblast differentiation by a mechanism independent of PKA activation (pentoxifylline promotes osteoblast differentiation). Endocrinology. 2001; 142(11):4673–4682. PMID: 11606432.51. McLeod NM, Pratt CA, Mellor TK, Brennan PA. Pentoxifylline and tocopherol in the management of patients with osteoradionecrosis, the Portsmouth experience. Br J Oral Maxillofac Surg. 2012; 50(1):41–44. PMID: 21247671.
Article52. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009; 122(2 Suppl):S14–S21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts of Vitamin D and Calcium in the Healing of Fractures
- The effect of two phosphodiesterase inhibitors on bone healing in mandibular fractures (animal study in rats)
- Clinical Utility of Biochemical Marker of Bone Turnover: Fracture Risk Prediction and Bone Healing
- Anti-osteoporotic Drugs and Fracture Healing Mechanism
- The Effect of Pentoxifylline on Rat Spinal Cord Damage to Fractionated Irradiation