Blood Res.
2017 Mar;52(1):18-24. 10.5045/br.2017.52.1.18.
Enhanced differentiation of mesenchymal stromal cells by three-dimensional culture and azacitidine
- Affiliations
-
- 1Laboratory of Hematological Disease and Immunology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yoojink@catholic.ac.kr
- 2Leukemia Research Institute, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Hematology, Catholic Blood and Marrow Transplantation Center, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2375199
- DOI: http://doi.org/10.5045/br.2017.52.1.18
Abstract
- BACKGROUND
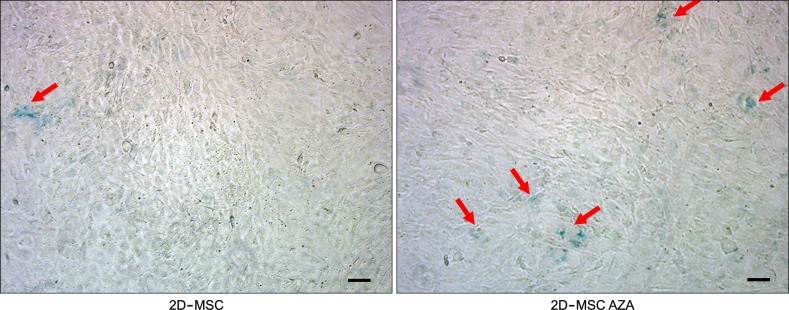
Mesenchymal stromal cells (MSCs) are useful for cell therapy because of their potential for multilineage differentiation. However, MSCs that are expanded in traditional two-dimensional (2D) culture systems eventually lose their differentiation abilities. Therefore, we investigated whether azacitidine (AZA) supplementation and three-dimensional culture (3D) could improve the differentiation properties of MSCs.
METHODS
2D- or 3D-cultured MSCs which were prepared according to the conventional or hanging-drop culture method respectively, were treated with or without AZA (1 µM for 72 h), and their osteogenic and adipogenic differentiation potential were determined and compared.
RESULTS
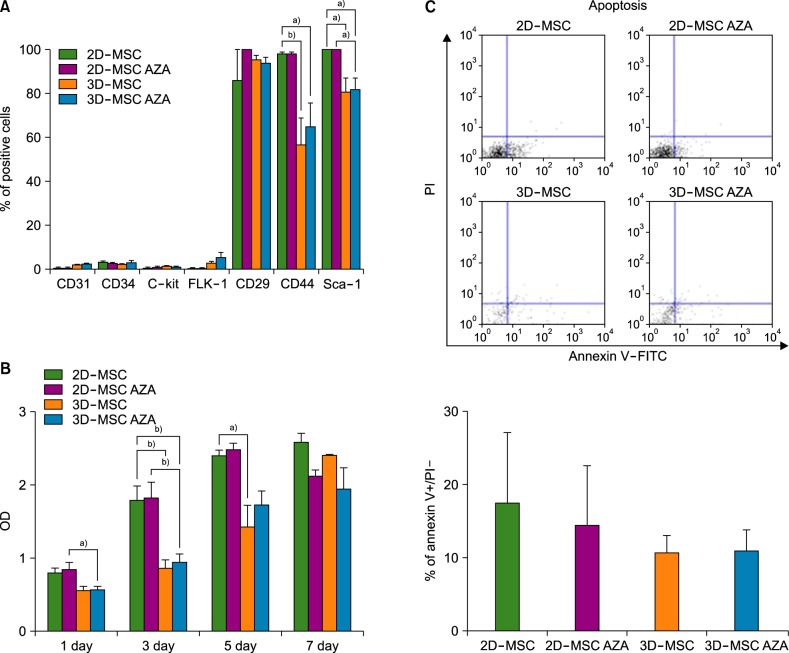
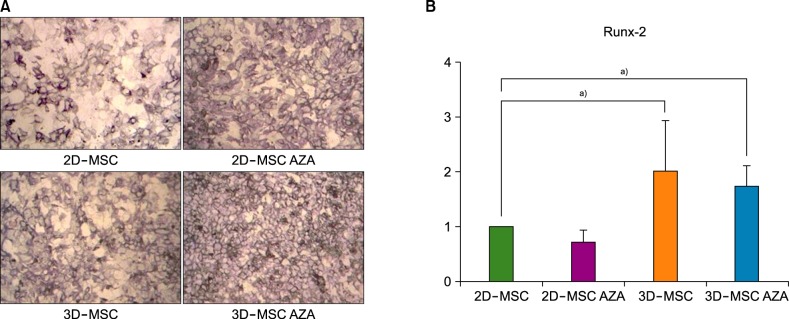
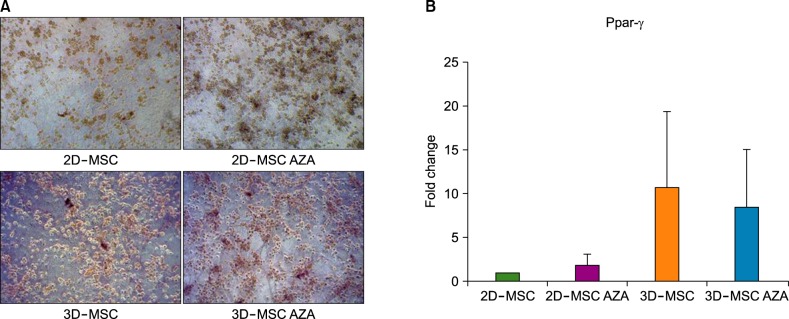
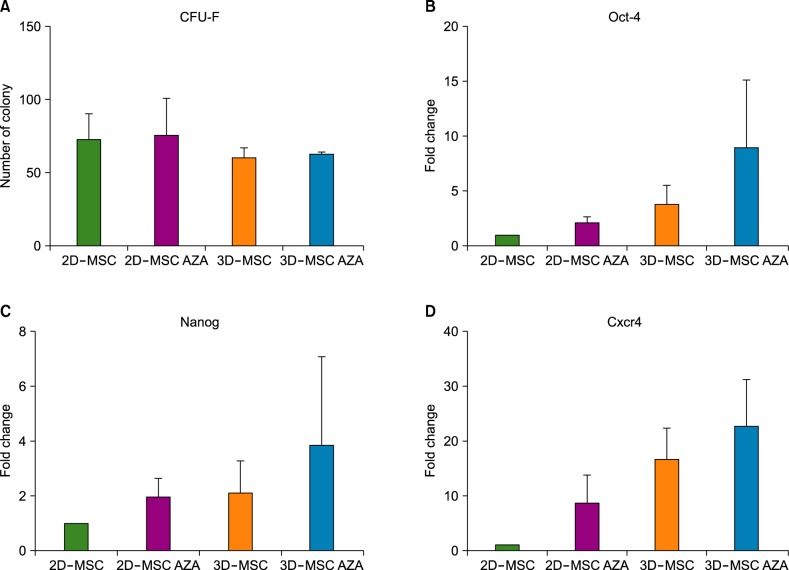
AZA treatment did not affect the cell apoptosis or viability in both 2D- and 3D-cultured MSCs. However, compared to conventionally cultured 2D-MSCs, AZA-treated 2D-MSCs showed marginally increased differentiation abilities. In contrast, 3D-MSCs showed significantly increased osteogenic and adipogenic differentiation ability. When 3D culture was performed in the presence of AZA, the osteogenic differentiation ability was further increased, whereas adipogenic differentiation was not affected.
CONCLUSION
3D culture efficiently promoted the multilineage differentiation of MSCs, and in combination with AZA, it could help MSCs to acquire greater osteogenic differentiation ability. This optimized culture method can enhance the therapeutic potential of MSCs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Alternative approaches to preserve MSC progenitor potency
Nayoun Kim, Seok-Goo Cho
Blood Res. 2017;52(1):1-2. doi: 10.5045/br.2017.52.1.1.
Reference
-
1. Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006; 13:419–425. PMID: 17053453.
Article2. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. PMID: 10102814.
Article3. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006; 7:14. PMID: 16529651.
Article4. Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005; 40:926–930. PMID: 16125890.
Article5. Papadimitropoulos A, Piccinini E, Brachat S, et al. Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion. PLoS One. 2014; 9:e102359. PMID: 25020062.
Article6. Rettinger CL, Fourcaudot AB, Hong SJ, Mustoe TA, Hale RG, Leung KP. In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res. 2014; 358:395–405. PMID: 25012521.
Article7. Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2014; 3:643–652. PMID: 24682286.
Article8. Bartosh TJ, Ylostalo JH. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr Protoc Stem Cell Biol. 2014; 28:Unit 2B.6. PMID: 24510769.
Article9. Kim HJ, Im GI. Electroporation-mediated transfer of SOX trio genes (SOX-5, SOX-6, and SOX-9) to enhance the chondrogenesis of mesenchymal stem cells. Stem Cells Dev. 2011; 20:2103–2114. PMID: 21401405.10. Huang B, Li G, Jiang XH. Fate determination in mesenchymal stem cells: a perspective from histone-modifying enzymes. Stem Cell Res Ther. 2015; 6:35. PMID: 25890062.
Article11. Ozkul Y, Galderisi U. The impact of epigenetics on mesenchymal stem cell biology. J Cell Physiol. 2016; 231:2393–2401. PMID: 26960183.
Article12. Kwon YR, Son MJ, Kim HJ, Kim YJ. Reactivation of silenced WT1 transgene by hypomethylating agents - implications for in vitro modeling of chemoimmunotherapy. Immune Netw. 2012; 12:58–65. PMID: 22740791.13. Kim HJ, Kwon YR, Bae YJ, Kim YJ. Enhancement of human mesenchymal stem cell differentiation by combination treatment with 5-azacytidine and trichostatin A. Biotechnol Lett. 2016; 38:167–174. PMID: 26341652.
Article14. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976; 4:267–274. PMID: 976387.15. Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000; 28:707–715. PMID: 10880757.16. Quito FL, Beh J, Bashayan O, Basilico C, Basch RS. Effects of fibroblast growth factor-4 (k-FGF) on long-term cultures of human bone marrow cells. Blood. 1996; 87:1282–1291. PMID: 8608216.
Article17. van de Kamp J, Paefgen V, Woltje M, et al. Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor-loaded biomaterials. J Tissue Eng Regen Med. 2016; [Epub ahead of print].
Article18. Cesarz Z, Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016; 2016:9176357. PMID: 26649054.
Article19. Kang PL, Chen CH, Chen SY, et al. Nano-sized collagen I molecules enhanced the differentiation of rat mesenchymal stem cells into cardiomyocytes. J Biomed Mater Res A. 2013; 101:2808–2816. PMID: 23463713.
Article20. Nakatsuka R, Nozaki T, Uemura Y, et al. 5-Aza-2'-deoxycytidine treatment induces skeletal myogenic differentiation of mouse dental pulp stem cells. Arch Oral Biol. 2010; 55:350–357. PMID: 20362276.
Article21. Xu S, De Veirman K, Evans H, et al. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol Sin. 2013; 34:699–709. PMID: 23564084.
Article22. Rosca AM, Burlacu A. Effect of 5-azacytidine: evidence for alteration of the multipotent ability of mesenchymal stem cells. Stem Cells Dev. 2011; 20:1213–1221. PMID: 21067364.
Article23. Jaenisch R, Schnieke A, Harbers K. Treatment of mice with 5-azacytidine efficiently activates silent retroviral genomes in different tissues. Proc Natl Acad Sci U S A. 1985; 82:1451–1455. PMID: 2579397.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars
- Coculture Effects on the Osteogenic Differentiation of Human Mesenchymal Stromal Cells
- Effective Keratocyte Culture Using Amniotic Membrane Matrix and Differentiation of Mesenchymal Stem Cells
- Cardiomyogenic Potential of Human Adipose Tissue and Umbilical Cord Derived-Mesenchymal Like Stem Cells
- Osteogenic Differentiation of Human Mesenchymal Stem Cells: Effects of the compositions of biodegradable polymers and the initial cell-seeding density