J Korean Med Sci.
2017 May;32(5):810-816. 10.3346/jkms.2017.32.5.810.
A Single Center Analysis of the Positivity of Hepatitis B Antibody after Neonatal Vaccination Program in Korea
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Hanyang University, Seoul, Korea. kyjoo@hanyang.ac.kr
- 2Database and Bioinformatics Laboratory, College of Electrical and Computer Engineering, Chungbuk National University, Cheongju, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, College of Medicine, Chungbuk National University, Cheongju, Korea.
- 4Biostatistics Consulting and Research Lab, College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 2375082
- DOI: http://doi.org/10.3346/jkms.2017.32.5.810
Abstract
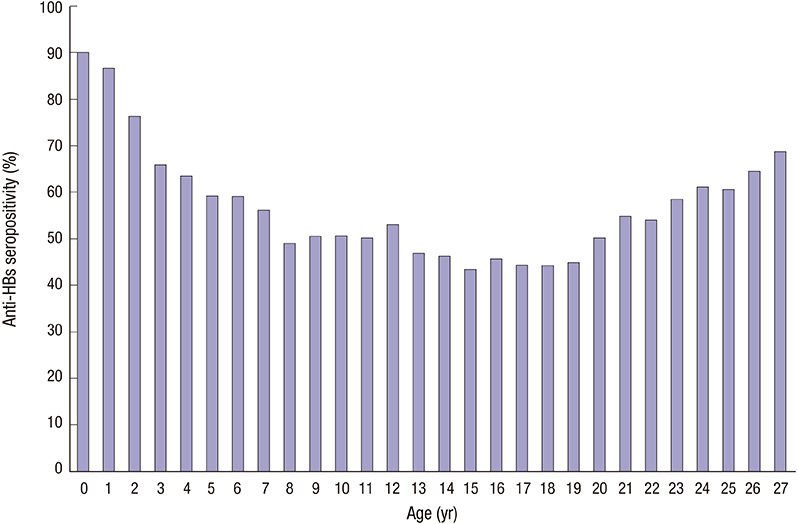
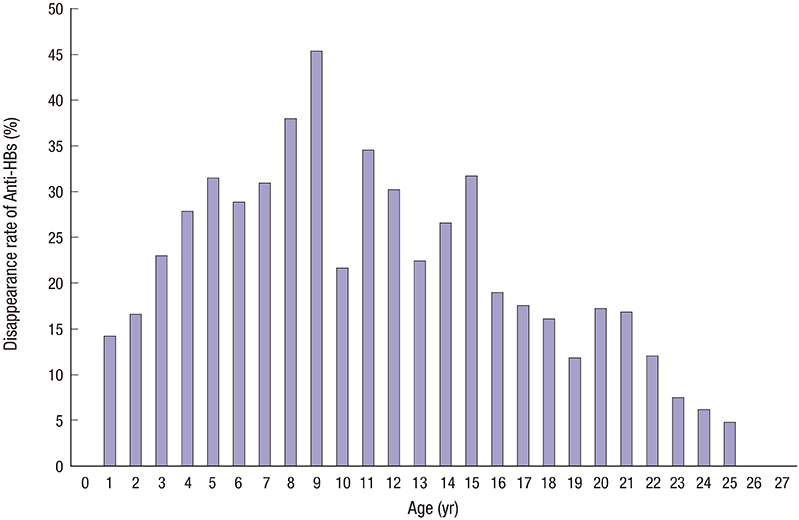
- The antibody to hepatitis B surface antigen (anti-HBs) seropositivity rate after 3 doses of hepatitis B virus (HBV) vaccination during infancy period is known to be higher than 90%. However, a considerable number of vaccines do not form protective anti-HBs or chronologic decrease of anti-HBs. We retrospectively collected data of HBV serologic test results in 20,738 individuals from 2000 to 2015. After exclusion criteria were applied, 19,072 individuals were included. We analyzed the anti-HBs seropositivity rate, anti-HBs disappearance rate, anti-HBs positive seroconversion rate after receiving a booster vaccine, and the difference in anti-HBs positivity between the 2 groups; group A (born before 2005, while both recombinant vaccines and plasma-derived vaccines were used) and group B (born after 2005, when only recombinant vaccines were used by national regulation). The anti-HBs seropositivity rate was 55.8%, but there was a significant difference in the rate of seropositivity for anti-HBs between the group A and B (53.0% vs. 78.1%, P < 0.001). There was no significant age-adjusted difference in the mean seropositivity rate between the 2 groups (P = 0.058). In addition, the anti-HBs positivity rate was significantly lower in the group A as compared with the group B during infancy (83.1% vs. 92.1%, P < 0.001). A total of 1,106 anti-HBs-positive subjects underwent serologic tests more than twice. Of these, 217 subjects (19.6%) showed anti-HBs disappearance. After booster vaccinations, 87.4% (83/95) achieved seroconversion from seronegative to seropositive. Our results highlight the importance of lifelong protection against HBV and the possible necessity of booster vaccination after adolescent period.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Significance of Decreasing Rate of HIV and HBV Co-infection in a Nationwide Korean HIV/AIDS Cohort
Yoonjung Kim, Shin-Woo Kim, Ki Tae Kwon, Hyun-Ha Chang, Yoonhee Jun, Jang Wook Sohn, Dae Won Park, Joon Young Song, Jun Yong Choi, Hyo Youl Kim, June Myung Kim, Bo Youl Choi, Yunsu Choi, Mee-Kyung Kee, Myeong Su Yoo, Jung Gyu Lee
J Korean Med Sci. 2020;35(3):. doi: 10.3346/jkms.2020.35.e7.
Reference
-
1. Chun BY, Lee MK, Rho YK. The prevalence of hepatitis B surface antigen among Korean by literature review. Korean J Epidemiol. 1992; 14:70–78.2. Choe YH, Seo JK, Yun JH, Lee HS. Recent changes in prevalence of hepatitis B viral markers in preschool children in Seoul, 1995. J Korean Pediatr Soc. 1996; 39:1254–1259.3. Jang MK, Lee JY, Lee JH, Kim YB, Kim HY, Yoo JY. The investigation for the change of HBsAg positive rate of grade junior high high-schoolers for recent 3 years in Kangwon province. Korean J Med. 2000; 58:608–615.4. Choe BH. The epidemiology and present status of chronic hepatitis B in Korean children. Korean J Pediatr. 2008; 51:696–703.5. Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, Holmberg SD. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016; 63:388–397.6. Park SH. Trends in the seroprevalence of hepatitis B surface antigen in the South Korean population. Int J Infect Dis. 2012; 16:e669–e672.7. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012; 4:74–80.8. Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010; 53:20–28.9. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002; 17:457–462.10. Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012; 57:730–735.11. Chen SM, Kung CM, Yang WJ, Wang HL. Efficacy of the nationwide hepatitis B infant vaccination program in Taiwan. J Clin Virol. 2011; 52:11–16.12. Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011; 53:68–75.13. McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005; 142:333–341.14. Kwon SY, Lee CH. Epidemiology and prevention of hepatitis B virus infection. Korean J Hepatol. 2011; 17:87–95.15. Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, Twu SJ, Su IJ, Huang LM, Lee CY. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004; 40:1415–1420.16. Chen TW. Paths toward hepatitis B immunization in South Korea and Taiwan. Clin Exp Vaccine Res. 2013; 2:76–82.17. Chung WK, Sun HS, Chung KW, Kim BS, Min BK, Prince AM. Safety and immunogenicity of a new heat-inactivated hepatitis B virus vaccine in adult recipients. Vaccine. 1987; 5:175–178.18. Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013; 10:239.19. Gringeri A. Factor VIII safety: plasma-derived versus recombinant products. Blood Transfus. 2011; 9:366–370.20. Hernández-Bernal F, Aguilar-Betancourt A, Aljovin V, Arias G, Valenzuela C, de Alejo KP, Hernández K, Oquendo O, Figueredo N, Figueroa N, et al. Comparison of four recombinant hepatitis B vaccines applied on an accelerated schedule in healthy adults. Hum Vaccin. 2011; 7:1026–1036.21. McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009; 200:1390–1396.22. Sim JG, Seo JK, Suh SJ. Prevalence and its changes of hepatitis B viral markers from 1988 to 1993 in Korean Children. J Korean Pediatr Soc. 1995; 38:1535–1539.23. Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY, et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007; 132:1287–1293.24. Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, Kim BH. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013; 28:413–419.25. Sung L, Heurter H, Zokvic KM, Ford-Jones EL, Weitzman SS, Freeman R, Dupuis LL, Scheifele DW. Practical vaccination guidelines for children with cancer. Paediatr Child Health. 2001; 6:379–383.26. Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum Vaccin Immunother. 2012; 8:896–904.27. Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012; 16:e82–e88.28. Yuen MF, Lim WL, Chan AO, Wong DK, Sum SS, Lai CL. 18-year follow-up study of a prospective randomized trial of hepatitis B vaccinations without booster doses in children. Clin Gastroenterol Hepatol. 2004; 2:941–945.29. Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine. 2009; 27:1858–1862.30. Lu JJ, Cheng CC, Chou SM, Hor CB, Yang YC, Wang HL. Hepatitis B immunity in adolescents and necessity for boost vaccination: 23 years after nationwide hepatitis B virus vaccination program in Taiwan. Vaccine. 2009; 27:6613–6618.31. Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005; 366:1379–1384.32. Jafarzadeh A, Montazerifar SJ. Persistence of anti-HBs antibody and immunological memory in children vaccinated with hepatitis B vaccine at birth. J Ayub Med Coll Abbottabad. 2006; 18:4–9.33. Cossio-Gil Y, Martínez-Gómez X, Campins-Martí M, Rodrigo-Pendás JÁ, Borruel-Sainz N, Rodríguez-Frías F, Casellas-Jordà F. Immunogenicity of hepatitis B vaccine in patients with inflammatory bowel disease and the benefits of revaccination. J Gastroenterol Hepatol. 2015; 30:92–98.34. Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev. 2000; 13:385–407.35. Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology. 2010; 51:1547–1554.36. Hartal M, Yavnai N, Galor I, Avramovich E, Sela T, Kayouf R, Tzurel-Ferber A, Greenberg LJ, Halperin T, Levine H. Seroprevalence of anti-HBs antibodies at young adulthood, before and after a booster vaccine dose, among medical personnel vaccinated in infancy. Vaccine. 2015; 33:4878–4885.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Antibody Formation After Hepatitis B Vaccination in Children
- Effect of Simultaneous Administration of Hepatitis B Vaccine with DPT and Oral Polio Vaccine
- The epidemiology and present status of chronic hepatitis B in Korean children
- Prevention of Viral Hepatitis and Vaccination
- Seroepidemiology of Hepatitis A and Hepatitis B in Korean Children