J Korean Neurosurg Soc.
2017 Feb;60(2):225-231. 10.3340/jkns.2017.0101.006.
Demineralized Bone Matrix (DBM) as a Bone Void Filler in Lumbar Interbody Fusion: A Prospective Pilot Study of Simultaneous DBM and Autologous Bone Grafts
- Affiliations
-
- 1Department of Neurosurgery, Korea University Ansan Hospital, Ansan, Korea. sehoonkim.ns@gmail.com
- KMID: 2374885
- DOI: http://doi.org/10.3340/jkns.2017.0101.006
Abstract
OBJECTIVE
Solid bone fusion is an essential process in spinal stabilization surgery. Recently, as several minimally invasive spinal surgeries have developed, a need of artificial bone substitutes such as demineralized bone matrix (DBM), has arisen. We investigated the in vivo bone growth rate of DBM as a bone void filler compared to a local autologous bone grafts.
METHODS
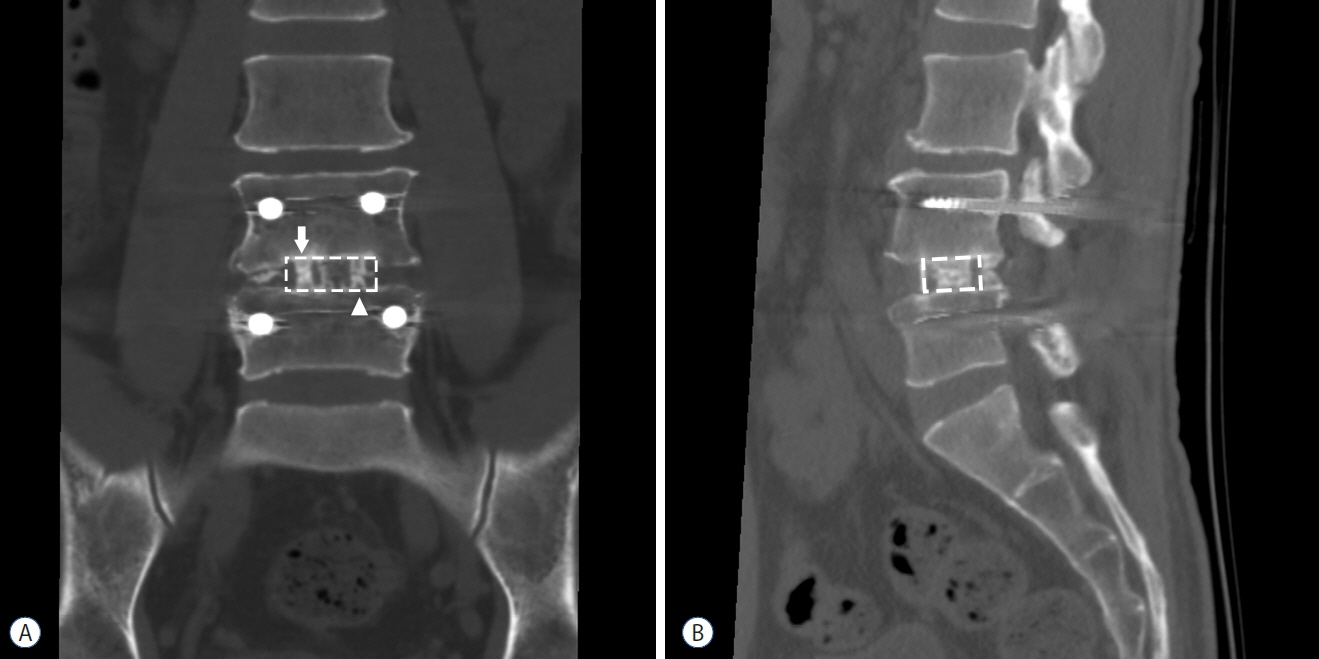
From April 2014 to August 2015, 20 patients with a one or two-level spinal stenosis were included. A posterior lumbar interbody fusion using two cages and pedicle screw fixation was performed for every patient, and each cage was packed with autologous local bone and DBM. Clinical outcomes were assessed using the Numeric Rating Scale (NRS) of leg pain and back pain and the Korean Oswestry Disability Index (K-ODI). Clinical outcome parameters and range of motion (ROM) of the operated level were collected preoperatively and at 3 months, 6 months, and 1 year postoperatively. Computed tomography was performed 1 year after fusion surgery and bone growth of the autologous bone grafts and DBM were analyzed by ImageJ software.
RESULTS
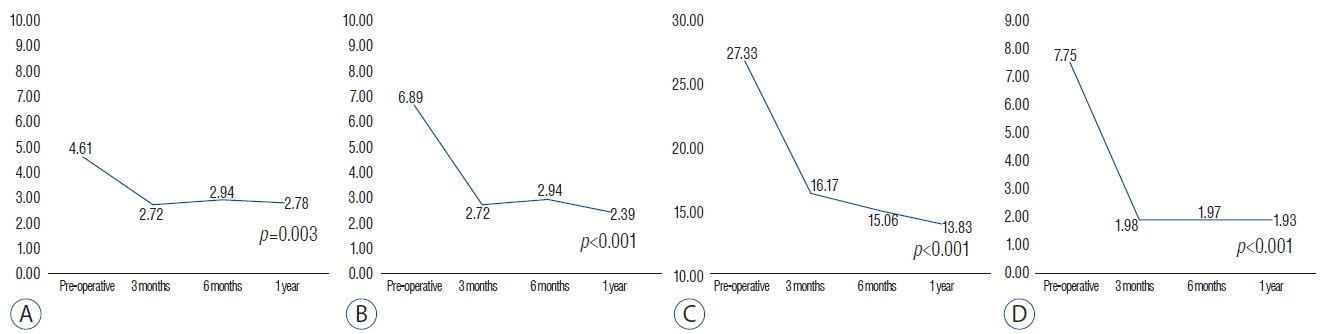
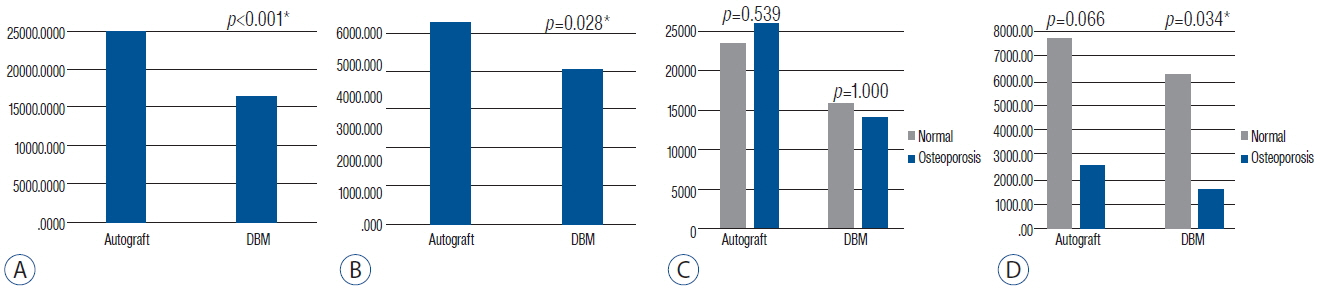
Eighteen patients completed 1 year of follow-up, including 10 men and 8 women, and the mean age was 56.4 (32-71). The operated level ranged from L3/4 to L5/S1. Eleven patients had single level and 7 patients had two-level repairs. The mean back pain NRS improved from 4.61 to 2.78 (p=0.003) and the leg pain NRS improved from 6.89 to 2.39 (p<0.001). The mean K-ODI score also improved from 27.33 to 13.83 (p<0.001). The ROM decreased below 2.0 degrees at the 3-month assessment, and remained less than 2 degrees through the 1 year postoperative assessment. Every local autologous bone graft and DBM packed cage showed bone bridge formation. On the quantitative analysis of bone growth, the autologous bone grafts showed significantly higher bone growth compared to DBM on both coronal and sagittal images (p<0.001 and p=0.028, respectively). Osteoporotic patients showed less bone growth on sagittal images.
CONCLUSION
Though DBM alone can induce favorable bone bridging in lumbar interbody fusion, it is still inferior to autologous bone grafts. Therefore, DBM is recommended as a bone graft extender rather than bone void filler, particularly in patients with osteoporosis.
Keyword
MeSH Terms
Figure
Reference
-
References
1. An HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. A prospective multicenter study. Spine (Phila Pa 1976). 20:2211–2216. 1995.
Article2. Boden SD, Schimandle JH. Biologic enhancement of spinal fusion. Spine (Phila Pa 1976). 20(24 Suppl):113S–123S. 1995.
Article3. Cammisa FP Jr, Lowery G, Garfin SR, Geisler FH, Klara PM, McGuire RA, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine (Phila Pa 1976). 29:660–666. 2004.
Article4. Chalmers J, Gray D, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 57:36–45. 1975.
Article5. Farrokhi MR, Rahmanian A, Masoudi MS. Posterolateral versus posterior interbody fusion in isthmic spondylolisthesis. J Neurotrauma. 29:1567–1573. 2012.
Article6. Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 84:454–464. 2002.
Article7. Fogel GR, Toohey JS, Neidre A, Brantigan JW. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 8:570–577. 2008.
Article8. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 36(Suppl 3):S20–S27. 2005.
Article9. Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 64:1063–1077. 2012.
Article10. He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, Chan CW, et al. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone. 48:1388–1400. 2011.
Article11. Kanayama M, Cunningham BW, Weis JC, Parker LM, Kaneda K, McAfee PC. The effects of rigid spinal instrumentation and solid bony fusion on spinal kinematics: a posterolateral spinal arthrodesis model. Spine (Phila Pa 1976). 23:767–773. 1998.
Article12. Kim DY, Lee SH, Lee HY, Lee HJ, Chang SB, Chung SK, et al. Validation of the Korean version of the oswestry disability index. Spine (Phila Pa 1976). 30:E123–E127. 2005.
Article13. Kim DH, Lee N, Shin DA, Yi S, Kim KN, Ha Y. Matched Comparison of Fusion Rates between Hydroxyapatite Demineralized Bone Matrix and Autograft in Lumbar Interbody Fusion. J Korean Neurosurg Soc. 59:363–367. 2016.
Article14. Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, et al. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 68:197–202. 1999.
Article15. Lang P, Genant HK, Chafetz N, Steiger P, Morris JM. Three-dimensional computed tomography and multiplanar reformations in the assessment of pseudarthrosis in posterior lumbar fusion patients. Spine (Phila Pa 1976). 13:69–75. 1988.
Article16. Larsen JM, Rimoldi RL, Capen DA, Nelson RW, Nagelberg S, Thomas JC Jr. Assessment of pseudarthrosis in pedicle screw fusion: a prospective study comparing plain radiographs, flexion/extension radiographs, CT scanning, and bone scintigraphy with operative findings. J Spinal Disord. 9:117–120. 1996.17. Lee KJ, Roper JG, Wang JC. Demineralized bone matrix and spinal arthrodesis. Spine J. 5(6 Suppl):S217–S223. 2005.
Article18. Lind M, Bünger C. Factors stimulating bone formation. Eur Spine J. 10(Suppl 2):S102–S109. 2001.
Article19. Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine (Phila Pa 1976). 23:159–167. 1998.
Article20. Najeeb S, Khurshid Z, Zohaib S, Zafar MS. Bioactivity and osseointegration of PEEK are inferior to those of titanium-a systematic review. J Oral Implantol. 42:512–516. 2016.
Article21. Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 8:457–470. 2009.
Article22. Ponnappan RK, Serhan H, Zarda B, Patel R, Albert T, Vaccaro AR. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation. Spine J. 9:263–267. 2009.
Article23. Sagomonyants KB, Jarman-Smith ML, Devine JN, Aronow MS, Gronowicz GA. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials. 29:1563–1572. 2008.
Article24. Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976). 28:997–1001. 2003.
Article25. Torricelli P, Fini M, Giavaresi G, Giardino R. In vitro osteoinduction of demineralized bone. Artif Cells Blood Substit Immobil Biotechnol. 26:309–315. 1998.
Article26. Torricelli P, Fini M, Rocca M, Giavaresi G, Giardino R. Xenogenic demineralized bone matrix: osteoinduction and influence of associated skeletal defects in heterotopic bone formation in rats. Int Orthop. 23:178–181. 1999.
Article27. Urist MR. Bone: formation by autoinduction. Science. 150:893–899. 1965.
Article28. Vadapalli S, Sairyo K, Goel VK, Robon M, Biyani A, Khandha A, et al. Biomechanical rationale for using polyetheretherketone (PEEK) spacers for lumbar interbody fusion–a finite element study. Spine (Phila Pa 1976). 31:E992–E998. 2006.
Article29. Young PM, Berquist TH, Bancroft LW, Peterson JJ. Complications of spinal instrumentation. Radiographics. 27:775–789. 2007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Demineralized Bone Matrix, as a Graft Enhancer of Auto-Local Bone in Posterior Lumbar Interbody Fusion
- Comparison of Fusion Rate between Demineralized Bone Matrix versus Autograft in Lumbar Fusion : Meta-Analysis
- Comparison of Clinical and Radiological Outcomes of Lumbar Interbody Fusion Using a Combination of Hydroxyapatite and Demineralized Bone Matrix and Autografts for Lumbar Degenerative Spondylolisthesis
- Bone Union Rate Following Instrumented Posterolateral Lumbar Fusion: Comparison between Demineralized Bone Matrix versus Hydroxyapatite
- The Effect of Demineralized Bone Matrix as a Graft Enhancer in Posterior Lumbar Interbody Fusion Using Cage and Local Bone Chips