J Korean Neurosurg Soc.
2017 Feb;60(2):130-137. 10.3340/jkns.2016.0101.006.
Memantine Induces NMDAR1-Mediated Autophagic Cell Death in Malignant Glioma Cells
- Affiliations
-
- 1Department of Neurosurgery, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea.
- 2Clinical Research Laboratory, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea.
- 3Institute of Catholic Integrative Medicine, Incheon St. Mary’s Hospital, The Catholic University of Korea, Incheon, Korea.
- 4Department of Neurosurgery, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ssjeun@catholic.ac.kr
- KMID: 2374873
- DOI: http://doi.org/10.3340/jkns.2016.0101.006
Abstract
OBJECTIVE
Autophagy is one of the key responses of cells to programmed cell death. Memantine, an approved anti-dementia drug, has an antiproliferative effect on cancer cells but the mechanism is poorly understood. The aim of the present study was to test the possibility of induction of autophagic cell death by memantine in glioma cell lines.
METHODS
Glioma cell lines (T-98 G and U-251 MG) were used for this study.
RESULTS
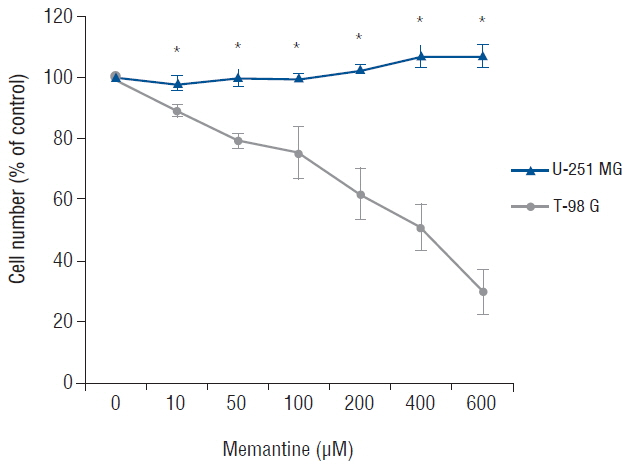
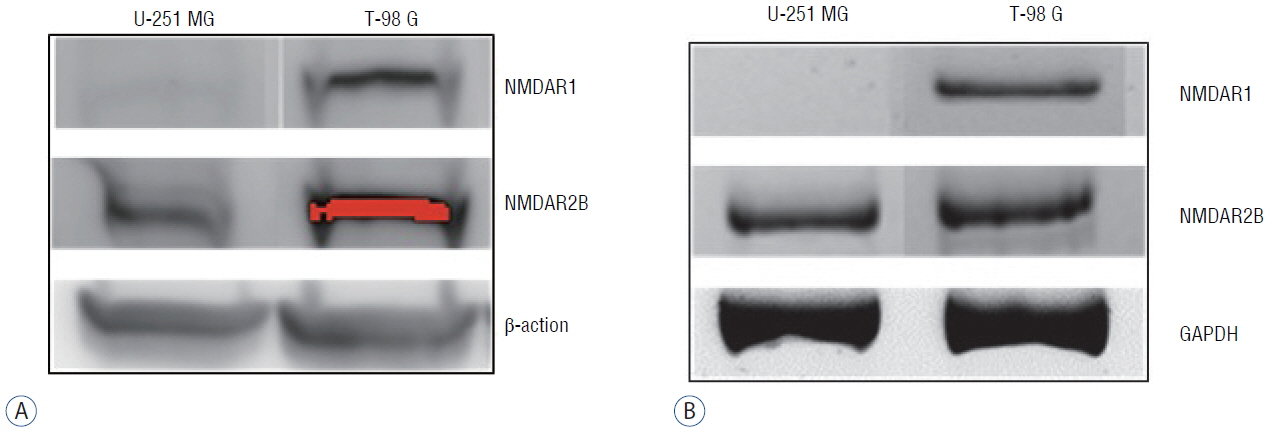
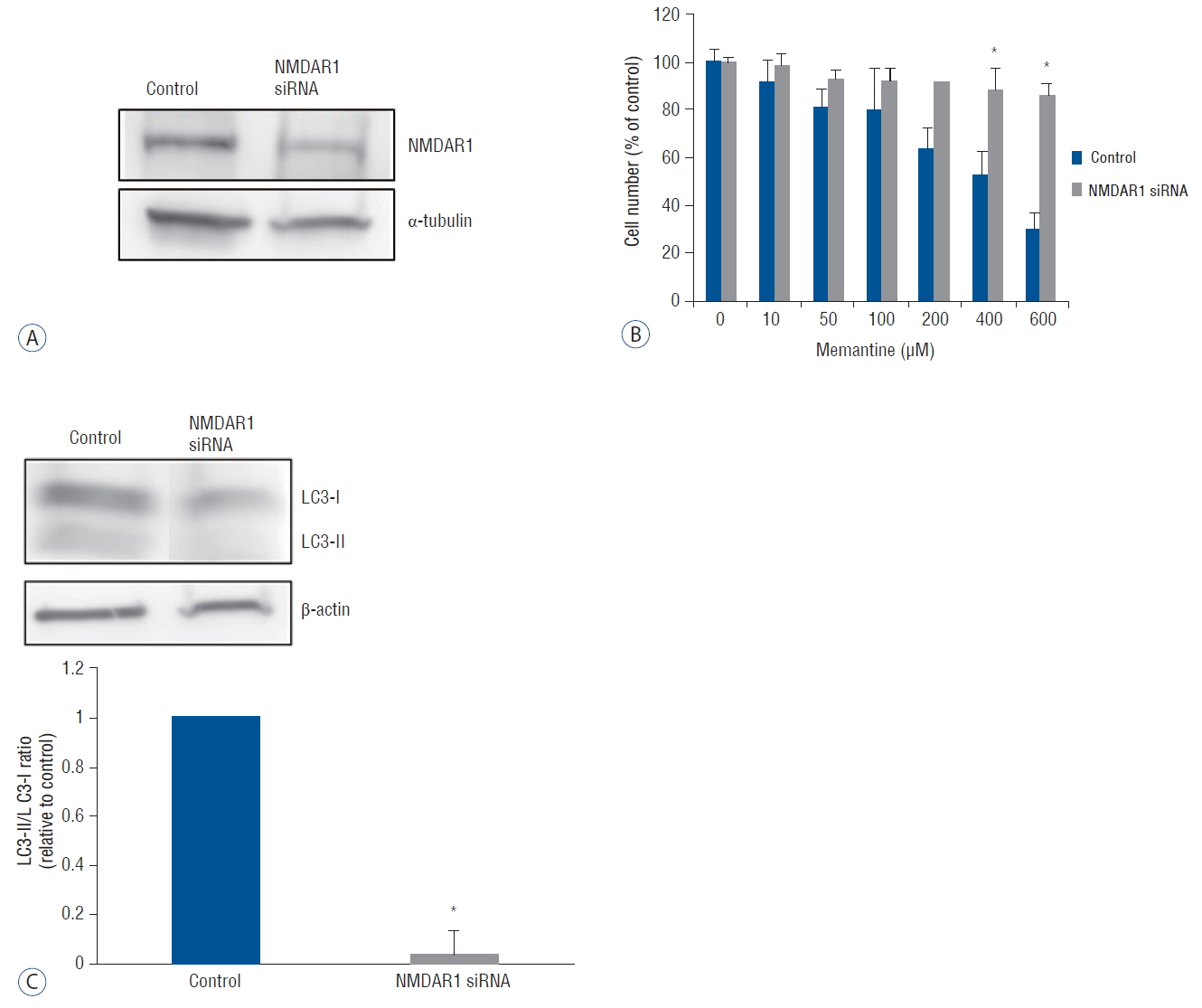
The antiproliferative effect of memantine was shown on T-98 G cells, which expressed N-methyl-D-aspartate 1 receptor (NMDAR1). Memantine increased the autophagic-related proteins as the conversion ratio of light chain protein 3-II (LC3-II)-/LC3-I and the expression of beclin-1. Memantine also increased formation of autophagic vacuoles observed under a transmission electron microscope. Transfection of small interfering RNA (siRNA) to knock down NMDAR1 in the glioma cells induced resistance to memantine and decreased the LC3-II/LC3-I ratio in T-98 G cells.
CONCLUSION
Our study demonstrates that in glioma cells, memantine inhibits proliferation and induces autophagy mediated by NMDAR1.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
N -retinylidene-N -retinylethanolamine degradation in human retinal pigment epithelial cellsvia memantine- and ifenprodil-mediated autophagy
Jae Rim Lee, Kwang Won Jeong
Korean J Physiol Pharmacol. 2023;27(5):449-456. doi: 10.4196/kjpp.2023.27.5.449.
Reference
-
References
1. Abdul M, Hoosein N. N-methyl-D-aspartate receptor in human prostate cancer. J Membr Biol. 205:125–128. 2005.
Article2. Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 18:1061–1083. 2009.
Article3. Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 47:11–22. 2000.4. Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and postsynaptic densities following transient global ischemia in the rat brain. J Neurochem. 93:186–194. 2005.
Article5. Bigford GE, Alonso OF, Dietrich D, Keane RW. A novel protein complex in membrane rafts linking the NR2B glutamate receptor and autophagy is disrupted following traumatic brain injury. J Neurotrauma. 26:703–720. 2009.
Article6. Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q, Feng XY, et al. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke. 39:3042–3048. 2008.
Article7. de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia. 59:1181–1189. 2011.
Article8. de Groot JF, Piao Y, Lu L, Fuller GN, Yung WK. Knockdown of GluR1 expression by RNA interference inhibits glioma proliferation. J Neurooncol. 88:121–133. 2008.
Article9. Fan G, Sun B, Wu Z, Guo Q, Guo Y. In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin Radiol. 59:77–85. 2004.
Article10. Ishiuchi S, Yoshida Y, Sugawara K, Aihara M, Ohtani T, Watanabe T, et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J Neurosci. 27:7987–8001. 2007.
Article11. Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 6:61–67. 2006.
Article12. Kihara K, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2:330–335. 2001.
Article13. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 8:445–544. 2012.14. Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 27:2846–2857. 2007.
Article15. McComb RD, Burger PC. Pathologic analysis of primary brain tumors. Neurol Clin. 3:711–728. 1985.
Article16. Moots PL, Maciunas RJ, Eisert DR, Parker RA, Laporte K, Abou-Khalil B. The course of seizure disorders in patients with malignant gliomas. Arch Neurol. 52:717–724. 1995.
Article17. North WG, Gao G, Jensen A, Memoli VA, Du J. NMDA receptors are expressed by small-cell lung cancer and are potential targets for effective treatment. Clin Pharmacol. 2:31–40. 2010.18. Piao Y, Lu L, de Groot J. AMPA receptors promote perivascular glioma invasion via beta1 integrin dependent adhesion to the extracellular matrix. Neuro Oncol. 11:260–273. 2009.
Article19. Rijpkema M, Schuuring J, van der MY, van der GM, Bernsen H, Boerman R, et al. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 16:12–18. 2003.
Article20. Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor–still lethal after eight years. Trends Neurosci. 18:57–58. 1995.21. Ruban A, Berkutzki T, Cooper I, Mohar B, Teichberg VI. Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas. Invest New Drugs. 30:2226–2235. 2012.
Article22. Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 98:6372–6377. 2001.
Article23. Schunemann DP, Grivicich I, Regner A, Leal LF, de Araujo DR, Jotz GP, et al. Glutamate promotes cell growth by EGFR signaling on U-87MG human glioblastoma cell line. Pathol Oncol Res. 16:285–293. 2010.
Article24. Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem. 105:287–295. 2008.
Article25. Stepulak A, Sifringer M, Rzeski W, Endesfelder S, Gratopp A, Pohl EE, et al. NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc Natl Acad Sci USA. 102:15605–15610. 2005.
Article26. Surawicz TS, Davis F, Freels S, Laws ER Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 40:151–160. 1998.27. Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 7:1010–1015. 2001.
Article28. Watanabe K, Kanno T, Oshima T, Miwa H, Tashiro C, Nishizaki T. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Biophys Res Commun. 367:487–490. 2008.
Article29. Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 3:109–110. 2004.
Article30. Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 59:4383–4391. 1999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Immunotherapeutic Approaches for Malignant Gliomas
- Radiation-Induced Autophagy Contributes to Cell Death and Induces Apoptosis Partly in Malignant Glioma Cells
- N-retinylidene-N-retinylethanolamine degradation in human retinal pigment epithelial cells via memantine- and ifenprodil-mediated autophagy
- Cell Death and Immunity
- in vitro Biological Response of Malignant Glioma Cell Lines to Gamma Knife Irradiation