J Breast Cancer.
2013 Mar;16(1):97-103.
Validation of a Web-Based Tool to Predict the Ipsilateral Breast Tumor Recurrence (IBTR! 2.0) after Breast-Conserving Therapy for Korean Patients
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Department of Surgery, Korea University Hospital, Korea University College of Medicine, Seoul, Korea.
- 2Department of Surgery, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 3Division of Breast and Endocrine Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jeongeon.lee@samsung.com
Abstract
- PURPOSE
IBTR! 2.0 is a web-based nomogram that predicts the 10-year ipsilateral breast tumor recurrence (IBTR) rate after breast-conserving therapy. We validated this nomogram in Korean patients.
METHODS
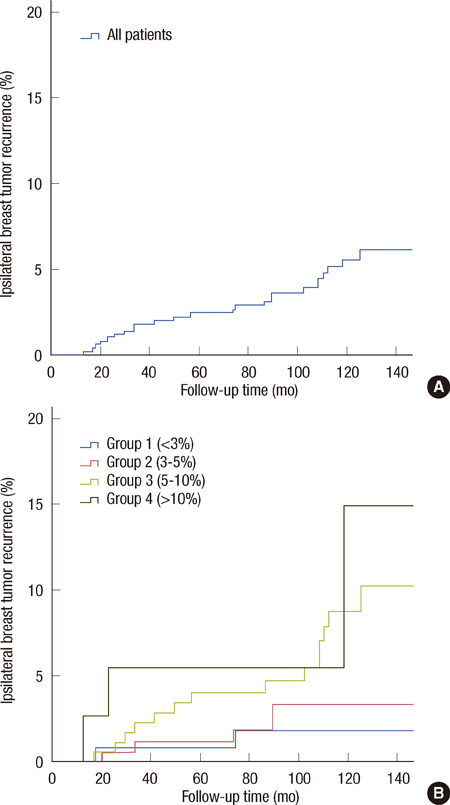
The nomogram was tested for 520 Korean patients, who underwent breast-conserving surgery followed by radiation therapy. Predicted and observed 10-year outcomes were compared for the entire cohort and for each group, predefined by nomogram-predicted risks: group 1, <3%; group 2, 3% to 5%; group 3, 5% to 10%; group 4, >10%.
RESULTS
In overall patients, the overall 10 year predicted and observed estimates of IBTR were 5.22% and 5.70% (p=0.68). In group 1, (n=124), the predicted and observed estimates were 2.25% and 1.80% (p=0.73), in group 2 (n=177), 3.95% and 3.90% (p=0.97), in group 3 (n=181), 7.14% and 8.80% (p=0.42), and in group 4 (n=38), 11.66% and 14.90% (p=0.73), respectively.
CONCLUSION
In a previous validation of this nomogram based on American patients, nomogram-predicted IBTR rates were overestimated in the high-risk subgroup. However, our results based on Korean patients showed that the observed IBTR was higher than the predicted estimates in groups 3 and 4. This difference may arise from ethnic differences, as well as from the methods used to detect IBTR and the healthcare environment. IBTR! 2.0 may be considered as an acceptable nomogram in Korean patients with low- to moderate-risk of in-breast recurrence. Before widespread use of this nomogram, the IBTR! 2.0 needs a larger validation study and continuous modification.
MeSH Terms
Figure
Reference
-
1. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 366:2087–2106.
Article2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.
Article3. Veronesi U, Marubini E, Mariani L, Galimberti V, Luini A, Veronesi P, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001. 12:997–1003.
Article4. Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabár L, Nordgren H, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999. 17:2326–2333.
Article5. Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008. 100:1179–1183.
Article6. Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001. 19:980–991.
Article7. Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992. 22:207–219.
Article8. Sanghani M, Truong PT, Raad RA, Niemierko A, Lesperance M, Olivotto IA, et al. Validation of a web-based predictive nomogram for ipsilateral breast tumor recurrence after breast conserving therapy. J Clin Oncol. 2010. 28:718–722.
Article9. Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009. 27:2466–2473.
Article10. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011. 43:1–11.
Article11. Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004. 22:4247–4254.
Article12. Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999. 17:1689–1700.
Article13. Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005. 23:2716–2725.
Article14. Keam B, Im SA, Park S, Nam BH, Han SW, Oh DY, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011. 137:1301–1308.
Article15. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996. 15:361–387.
Article16. Sanghani M, Balk E, Cady B, Wazer D. Predicting the risk of local recurrence in patients with breast cancer: an approach to a new computer-based predictive tool. Am J Clin Oncol. 2007. 30:473–480.17. Kunos C, Latson L, Overmoyer B, Silverman P, Shenk R, Kinsella T, et al. Breast conservation surgery achieving>or=2 mm tumor-free margins results in decreased local-regional recurrence rates. Breast J. 2006. 12:28–36.
Article18. Hanna WM, Kahn HJ, Chapman JA, Fish EB, Lickley HL, McCready DR. Pathologic characteristics of breast cancer that predict for local recurrence after lumpectomy alone. Breast J. 1999. 5:105–111.
Article19. Clark RM, Whelan T, Levine M, Roberts R, Willan A, McCulloch P, et al. Ontario Clinical Oncology Group. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst. 1996. 88:1659–1664.
Article20. Arriagada R, Lê MG, Contesso G, Guinebretière JM, Rochard F, Spielmann M. Predictive factors for local recurrence in 2006 patients with surgically resected small breast cancer. Ann Oncol. 2002. 13:1404–1413.
Article21. Kang SH, Chung KY, Kim YS, Kim JH. Disease free survival and prognostic factors for patients with breast conserving surgery. J Korean Surg Soc. 2004. 67:274–278.22. Kim JH, Han W, Moon HG, Ko E, Lee JW, Kim EK, et al. Factors affecting the ipsilateral breast tumor recurrence after breast conserving therapy in patients with T1 and T2 tumors. J Breast Cancer. 2009. 12:324–330.
Article23. Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002. 20:4141–4149.
Article24. Kim SH, Kim MH, Oh KK. Analysis and comparison of breast density according to age on mammogram between Korean and Western women. J Korean Radiol Soc. 2000. 42:1009–1014.
Article25. Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010. 102:1224–1237.
Article26. Statistics Korea. Birth statics 2010. Accessed October 1st, 2012. http://kostat.go.kr.27. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011. 60:1–70.28. Poortmans PM, Collette L, Horiot JC, Van den Bogaert WF, Fourquet A, Kuten A, et al. Impact of the boost dose of 10 Gy versus 26 Gy in patients with early stage breast cancer after a microscopically incomplete lumpectomy: 10-year results of the randomised EORTC boost trial. Radiother Oncol. 2009. 90:80–85.
Article29. Cheng SH, Horng CF, West M, Huang E, Pittman J, Tsou MH, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer. J Clin Oncol. 2006. 24:4594–4602.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Validation of a Web-Based Tool to Predict the Ipsilateral Breast Tumor Recurrence (IBTR! 2.0) after Breast-Conserving Therapy for Korean Patients
- Ipsilateral Breast Tumor Recurrence with Metachronous Contralateral Axillary Lymph Node Metastasis after Breast-Conserving Surgery with Axillary Lymph Node Dissection
- External validation of IBTR! 2.0 nomogram for prediction of ipsilateral breast tumor recurrence
- Surgical Options for Ipsilateral Breast Tumor Recurrence: Mastectomy Versus Repeat Breast-Conserving Surgery
- Recurrent and Second Breast Cancer Detected on Follow-Up Mammography and Breast Ultrasound after Breast-Conserving Surgery: Imaging Findings and Clinicopathologic Factors