J Breast Cancer.
2015 Jun;18(2):200-205. 10.4048/jbc.2015.18.2.200.
Primary Inflammatory Myofibroblastic Tumors of the Breast with Metastasis: Radiographic and Histopathologic Predictive Factors
- Affiliations
-
- 1Department of Radiology, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Institute for Medical Sciences of Chonbuk National University Medical School, Jeonju, K
- 2Department of Pathology, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Institute for Medical Sciences of Chonbuk National University Medical School, Jeonju, K
- 3Department of Surgery, Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Institute for Medical Sciences of Chonbuk National University Medical School, Jeonju, Kor
- KMID: 2374537
- DOI: http://doi.org/10.4048/jbc.2015.18.2.200
Abstract
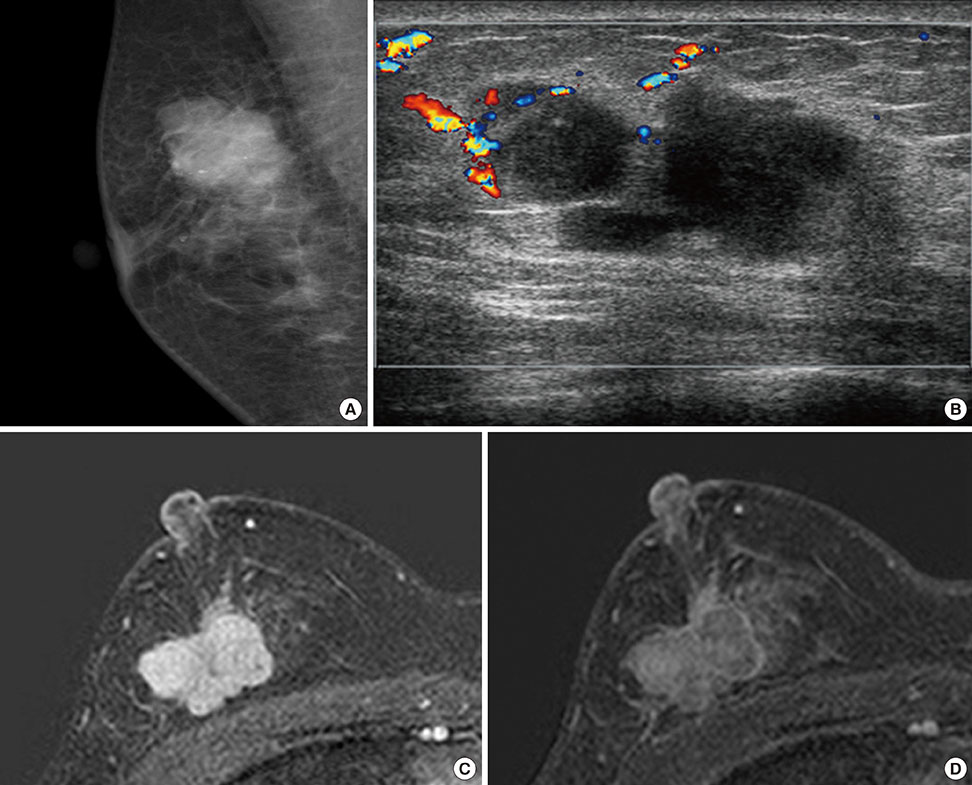
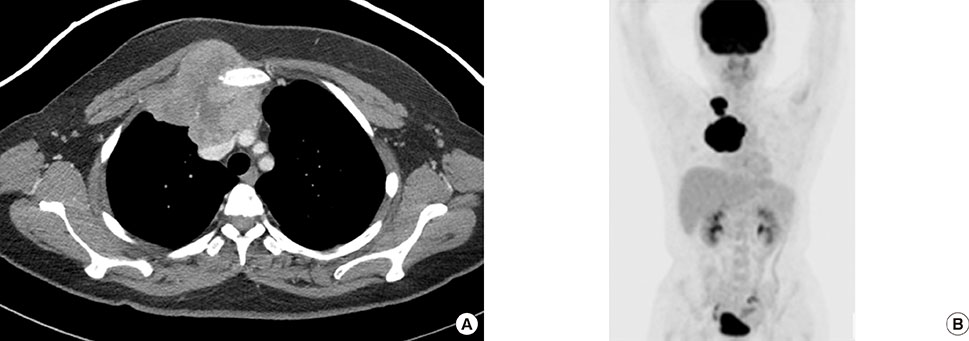
- Primary inflammatory myofibroblastic tumors (IMTs) of the breast are uncommon and metastasis of IMTs is extremely rare. To date, the natural course of this disease is not fully understood. Although patients with IMTs should undergo regular follow-up after complete surgical resection of the tumor, the appropriate interval and method of follow-up are unclear. We report the case of a patient with an IMT of the breast that metastasized 2 years after complete surgical resection. This unusual case emphasizes the importance of preoperative examinations to determine whether the IMT has atypical features that should guide the interval and method of follow-up.
Keyword
MeSH Terms
Figure
Reference
-
1. Haj M, Weiss M, Loberant N, Cohen I. Inflammatory pseudotumor of the breast: case report and literature review. Breast J. 2003; 9:423–425.
Article2. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007; 31:509–520.3. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995; 19:859–872.
Article4. Zhao HD, Wu T, Wang JQ, Zhang WD, He XL, Bao GQ, et al. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: a case report. Oncol Lett. 2013; 5:97–100.
Article5. Vecchio GM, Amico P, Grasso G, Vasquez E, La Greca G, Magro G. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: a potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract. 2011; 207:322–326.
Article6. Kim SJ, Moon WK, Kim JH, Cho N, Chang CM. Inflammatory pseudotumor of the breast: a case report with imaging findings. Korean J Radiol. 2009; 10:515–518.7. Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005; 113:66–69.
Article8. Sastre-Garau X, Couturier J, Derré J, Aurias A, Klijanienko J, Lagacé R. Inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast: clinicopathological and genetic analysis of a case with evidence for clonality. J Pathol. 2002; 196:97–102.
Article9. Trojan A, Stallmach T, Kollias S, Pestalozzi BC. Inflammatory myofibroblastic tumor with CNS involvement. Onkologie. 2001; 24:368–372.
Article10. Myint MA, Medeiros LJ, Sulaiman RA, Aswad BI, Glantz L. Inflammatory pseudotumor of the ileum: a report of a multifocal, transmural lesion with regional lymph node involvement. Arch Pathol Lab Med. 1994; 118:1138–1142.11. Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999; 12:279–286.12. Mergan F, Jaubert F, Sauvat F, Hartmann O, Lortat-Jacob S, Révillon Y, et al. Inflammatory myofibroblastic tumor in children: clinical review with anaplastic lymphoma kinase, Epstein-Barr virus, and human herpesvirus 8 detection analysis. J Pediatr Surg. 2005; 40:1581–1586.
Article13. Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, et al. 23-Year-old female with an inflammatory myofibroblastic tumour of the breast: a case report and a review of the literature. Geburtshilfe Frauenheilkd. 2014; 74:167–170.
Article14. Tse GM, Chaiwun B, Wong KT, Yeung DK, Pang AL, Tang AP, et al. Magnetic resonance imaging of breast lesions: a pathologic correlation. Breast Cancer Res Treat. 2007; 103:1–10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory Myofibroblastic Tumor of Nasal Septum after Septoplasty: A Case Report
- Inflammatory Myofibroblastic Tumor of the Breast: A Case Report
- Inflammatory Myofibroblastic Tumor of the Breast
- Inflammatory Myofibroblastic Tumor of the Breast
- Clinicopathological Study of 18 Cases of Inflammatory Myofibroblastic Tumors with Reference to ALK-1 Expression: 5-Year Experience in a Tertiary Care Center