Yonsei Med J.
2017 Jan;58(1):131-138. 10.3349/ymj.2017.58.1.131.
Hyperbaric Oxygen Pretreatment Improves Cognition and Reduces Hippocampal Damage Via p38 Mitogen-Activated Protein Kinase in a Rat Model
- Affiliations
-
- 1Department of Anesthesiology, Guangzhou Women and Children’s Medical Center, Guangzhou, China. songxingrong510623@163.com
- KMID: 2374198
- DOI: http://doi.org/10.3349/ymj.2017.58.1.131
Abstract
- PURPOSE
To investigate the effects of hyperbaric oxygen (HBO) pretreatment on cognitive decline and neuronal damage in an Alzheimer's disease (AD) rat model.
MATERIALS AND METHODS
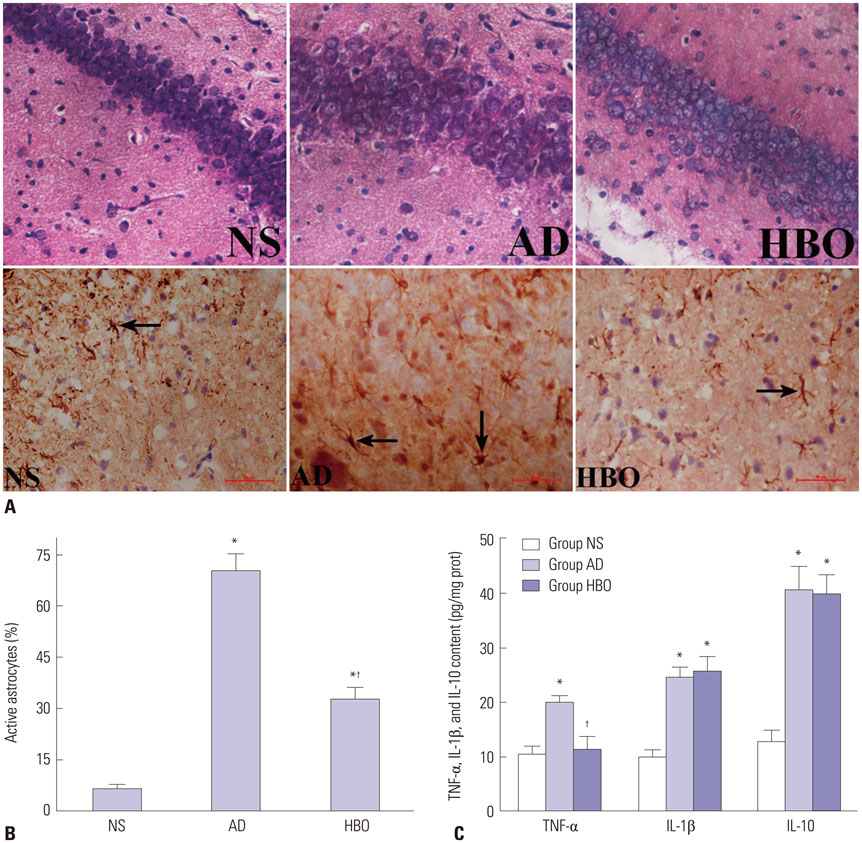
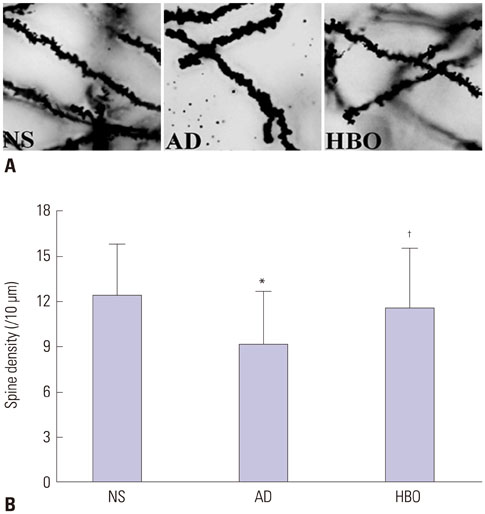
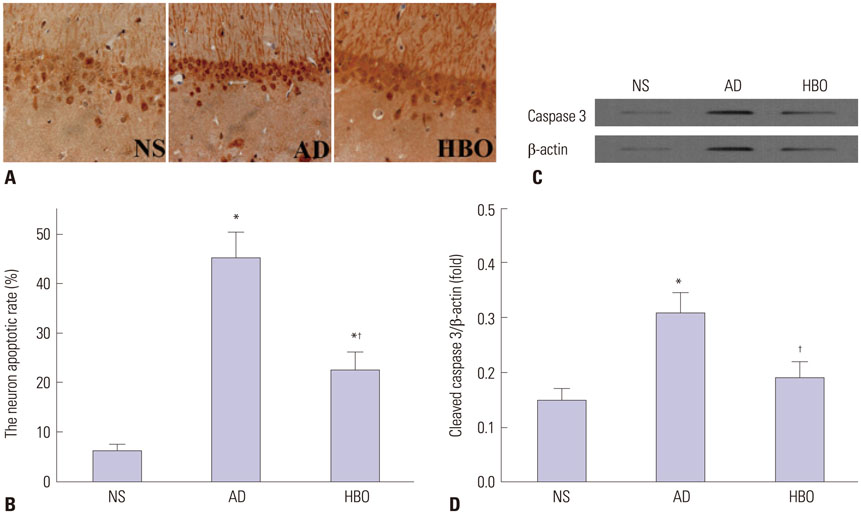
Rats were divided into three groups: normal saline (NS), AD, and HBO+AD. In the AD group, amyloid β peptide (Aβ)â‚â‚‹â‚„â‚€ was injected into the hippocampal CA1 region of the brain. NS rats received NS injection. In the HBO+AD group, rats received 5 days of daily HBO therapy following Aβâ‚â‚‹â‚„â‚€ injection. Learning and memory capabilities were examined using the Morris water maze task. Neuronal damage and astrocyte activation were evaluated by hematoxylin-eosin staining and immunohistochemistry, respectively. Dendritic spine density was determined by Golgi-Cox staining. Tumor necrosis factor-α, interleukin-1β, and interleukin-10 production was assessed by enzyme-linked immunosorbent assay. Neuron apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling. Protein expression was examined by western blotting.
RESULTS
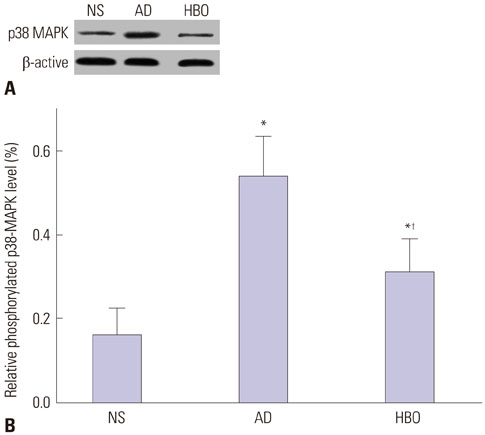
Learning and memory dysfunction was ameliorated in the HBO+AD group, as shown by significantly lower swimming distances and escape latency, compared to the AD group. Lower rates of neuronal damage, astrocyte activation, dendritic spine loss, and hippocampal neuron apoptosis were seen in the HBO+AD than in the AD group. A lower rate of hippocampal p38 mitogen-activated protein kinase (MAPK) phosphorylation was observed in the HBO+AD than in the AD group.
CONCLUSION
HBO pretreatment improves cognition and reduces hippocampal damage via p38 MAPK in AD rats.
Keyword
MeSH Terms
-
Alzheimer Disease/*therapy
Amyloid beta-Peptides/*administration & dosage
Animals
Apoptosis
*Cognition/drug effects
Disease Models, Animal
Enzyme-Linked Immunosorbent Assay
Hippocampus/*enzymology
*Hyperbaric Oxygenation
In Situ Nick-End Labeling
Interleukin-10/biosynthesis
Interleukin-1beta/biosynthesis
Learning/drug effects
Male
Memory/drug effects
Neurons
Peptide Fragments/*administration & dosage
Rats
Rats, Sprague-Dawley
Sodium Chloride/administration & dosage
Tumor Necrosis Factor-alpha/biosynthesis
p38 Mitogen-Activated Protein Kinases/*metabolism
Amyloid beta-Peptides
Interleukin-10
Interleukin-1beta
Peptide Fragments
Sodium Chloride
Tumor Necrosis Factor-alpha
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012; 307:1798–1800.
Article2. Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010; 37:503–509.
Article3. Zilka N, Ferencik M, Hulin I. Neuroinflammation in Alzheimer’s disease: protector or promoter? Bratisl Lek Listy. 2006; 107:374–383.4. Malm T, Ort M, Tähtivaara L, Jukarainen N, Goldsteins G, Puoliväli J, et al. beta-Amyloid infusion results in delayed and age-dependent learning deficits without role of inflammation or beta-amyloid deposits. Proc Natl Acad Sci U S A. 2006; 103:8852–8857.
Article5. Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology. 2010; 58:561–568.
Article6. Sun A, Liu M, Nguyen XV, Bing G. P38 MAP kinase is activated at early stages in Alzheimer’s disease brain. Exp Neurol. 2003; 183:394–405.
Article7. Corrêa SA, Eales KL. The Role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduct. 2012; 2012:649079.
Article8. Zhao BS, Song XR, Hu PY, Meng LX, Tan YH, She YJ, et al. Hyperbaric oxygen treatment at various stages following chronic constriction injury produces different antinociceptive effects via regulation of P2X4R expression and apoptosis. PLoS One. 2015; 10:e0120122.
Article9. Liu XH, Yan H, Xu M, Zhao YL, Li LM, Zhou XH, et al. Hyperbaric oxygenation reduces long-term brain injury and ameliorates behavioral function by suppression of apoptosis in a rat model of neonatal hypoxia-ischemia. Neurochem Int. 2013; 62:922–930.
Article10. Li F, Fang L, Huang S, Yang Z, Nandi J, Thomas S, et al. Hyperbaric oxygenation therapy alleviates chronic constrictive injury-induced neuropathic pain and reduces tumor necrosis factor-alpha production. Anesth Analg. 2011; 113:626–633.
Article11. Tian X, Zhang L, Wang J, Dai J, Shen S, Yang L, et al. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25-35-induced oxidative stress and neuronal apoptosis in rats. Behav Brain Res. 2013; 242:1–8.
Article12. Tian X, Wang J, Dai J, Yang L, Zhang L, Shen S, et al. Hyperbaric oxygen and Ginkgo Biloba extract inhibit Aβ25-35-induced toxicity and oxidative stress in vivo: a potential role in Alzheimer’s disease. Int J Neurosci. 2012; 122:563–569.
Article13. Zhao BS, Meng LX, Ding YY, Cao YY. Hyperbaric oxygen treatment produces an antinociceptive response phase and inhibits astrocyte activation and inflammatory response in a rat model of neuropathic pain. J Mol Neurosci. 2014; 53:251–261.
Article14. Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in β-amyloid rat model of Alzheimer’s disease. J Neuroinflammation. 2012; 9:202.15. Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M. Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiol Aging. 2013; 34:2843–2852.
Article16. Deng J, Qi XL, Guan ZZ, Yan XM, Huang Y, Wang YL. Pretreatment of SH-SY5Y cells with dicaffeoylquinic acids attenuates the reduced expression of nicotinic receptors, elevated level of oxidative stress and enhanced apoptosis caused by β-amyloid peptide. J Pharm Pharmacol. 2013; 65:1736–1744.
Article17. Yang Y, Chen S, Zhang J, Li C, Sun Y, Zhang L, et al. Stimulation of autophagy prevents amyloid-β peptide-induced neuritic degeneration in PC12 cells. J Alzheimers Dis. 2014; 40:929–939.
Article18. Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation. 2011; 8:150.
Article19. Rozkalne A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011; 41:650–654.
Article20. Song JM, DiBattista AM, Sung YM, Ahn JM, Turner RS, Yang J, et al. A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer’s disease. Exp Neurol. 2014; 252:105–113.
Article21. Wang R, Palavicini JP, Wang H, Maiti P, Bianchi E, Xu S, et al. RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2014; 69:169–179.
Article22. Gu N, Niu JY, Liu WT, Sun YY, Liu S, Lv Y, et al. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. Eur J Pain. 2012; 16:1094–1105.
Article23. Mori H, Oikawa M, Tamagami T, Kumaki H, Nakaune R, Amano J, et al. Oxidized proteins in astrocytes generated in a hyperbaric atmosphere induce neuronal apoptosis. J Alzheimers Dis. 2007; 11:165–174.
Article24. Park JG, Yuk Y, Rhim H, Yi SY, Yoo YS. Role of p38 MAPK in the regulation of apoptosis signaling induced by TNF-alpha in differentiated PC12 cells. J Biochem Mol Biol. 2002; 35:267–272.
Article25. Bühler S, Laufer SA. p38 MAPK inhibitors: a patent review (2012 - 2013). Expert Opin Ther Pat. 2014; 24:535–554.
Article26. Heyboer M 3rd, Jennings S, Grant WD, Ojevwe C, Byrne J, Wojcik SM. Seizure incidence by treatment pressure in patients undergoing hyperbaric oxygen therapy. Undersea Hyperb Med. 2014; 41:379–385.27. Obiagwu C, Paul V, Chadha S, Hollander G, Shani J. Acute pulmonary edema secondary to hyperbaric oxygen therapy. Oxf Med Case Reports. 2015; 2015:183–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitogen-activated Protein Kinases in the Development of Normal and Diseased Kidneys
- Effect of kainic acid on the phosphorylation of mitogen activated protein kinases in rat hippocampus
- Angiotensin Converting Enzyme Inhibition Modulates Mitogen Activated Protein Kinase Family Expressions in the Neonatal Rat Kidney
- Cadmium induces apoptosis in primary rat osteoblasts through caspase and mitogen-activated protein kinase pathways
- Effect of Mild Hypothermia on the Mitogen Activated Protein Kinases in Experimental Stroke