Yonsei Med J.
2017 Jan;58(1):43-50. 10.3349/ymj.2017.58.1.43.
Identificaiton of Novel Immunogenic Human Papillomavirus Type 16 E7-Specific Epitopes Restricted to HLA-A*33;03 for Cervical Cancer Immunotherapy
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. jlim@yuhs.ac
- KMID: 2374187
- DOI: http://doi.org/10.3349/ymj.2017.58.1.43
Abstract
- PURPOSE
To identify new immunogenic HLA-A*33;03-restricted epitopes from the human papillomavirus (HPV) 16 E7 protein for immunotherapy against cervical cancer.
MATERIALS AND METHODS
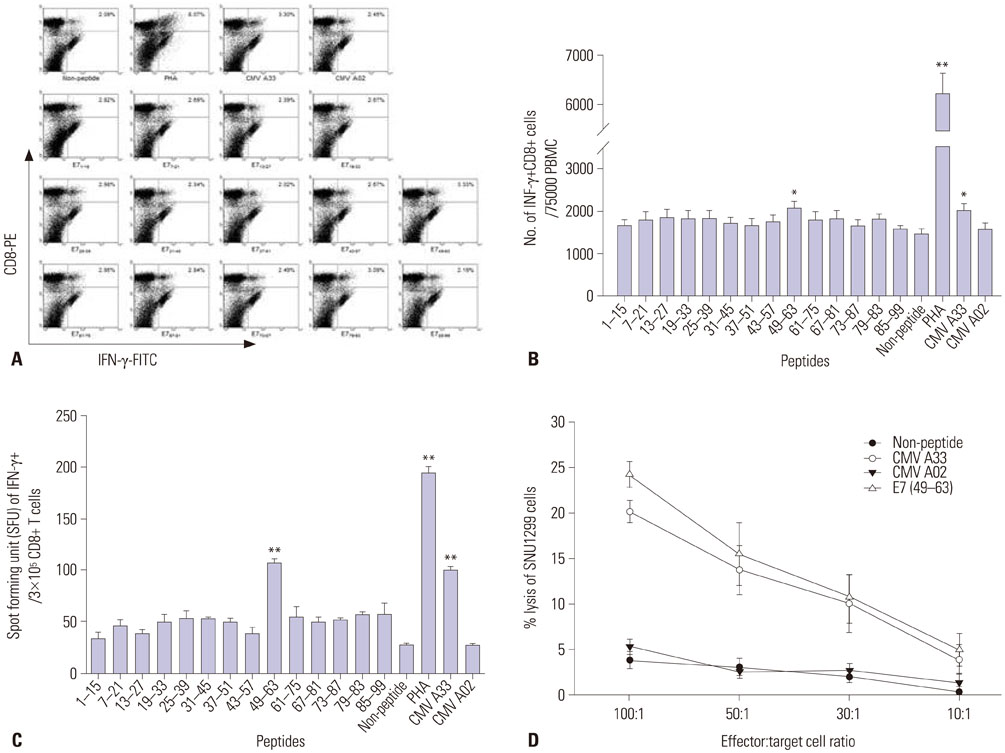
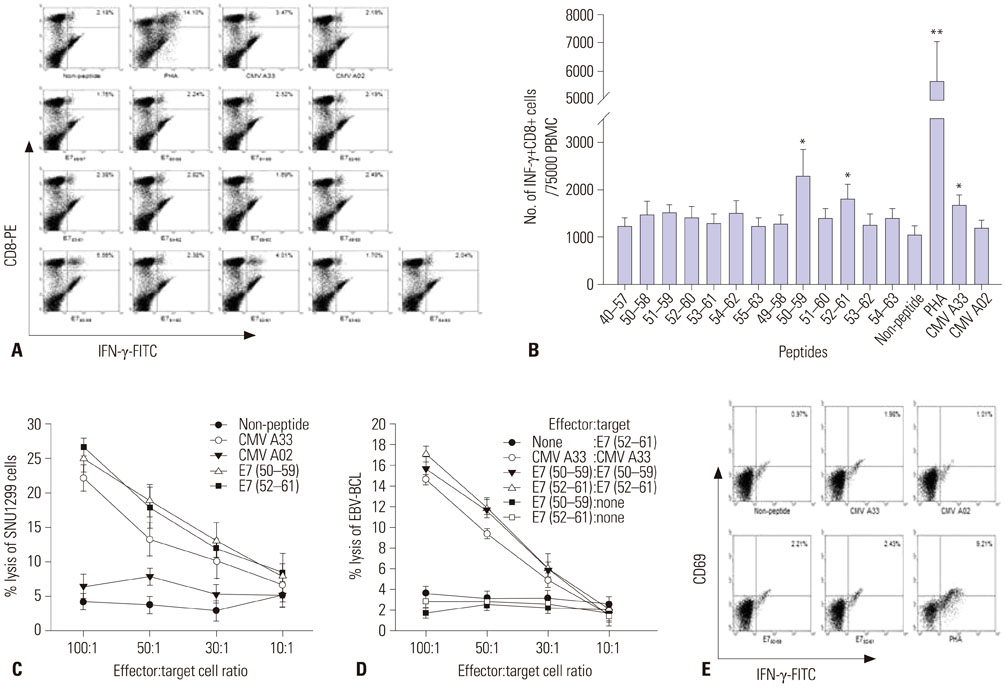
We synthesized fourteen overlapping 15-amino acid peptides and measured intracellular interferon-γ (IFN-γ) production in PBMC and CD8+ cytotoxic T lymphocytes (CTLs) after sensitization with these peptides using flow cytometry and ELISpot assay. The immunogenicity of epitopes was verified using a ⵹Cr release assay with SNU1299 cells.
RESULTS
Among the fourteen 15-amino acid peptides, E7₄₉₋₆₃ (RAHYNIVTFCCKCDS) demonstrated the highest IFN-γ production from peripheral blood mononuclear cells (PBMCs), and CD8+ CTLs sensitized with E7₄₉₋₆₃ showed higher cytotoxic effect against SNU1299 cells than did CD8+ CTLs sensitized with other peptides or a negative control group. Thirteen 9- or 10-amino acid overlapping peptides spanning E7₄₉₋₆₃, E7₅₀₋₅₉ (AHYNIVTFCC), and E7₅₂₋₆₠(YNIVTFCCKC) induced significantly higher IFN-γ production and cytotoxic effects against SNU1299 cells than the other peptides and negative controls, and the cytotoxicity of E7₅₀₋₅₉- and E7₅₂₋₆â‚-sensitized PBMCs was induced via the cytolytic effect of CD8+ CTLs.
CONCLUSION
We identified E7₅₀₋₅₉ and E7₅₂₋₆₠as novel HPV 16 E7 epitopes for HLA-A*33;03. CD8+ CTL sensitized with these peptides result in an antitumor effect against cervical cancer cells. These epitopes could be useful for immune monitoring and immunotherapy for cervical cancer and HPV 16-related diseases including anal cancer and oropharyngeal cancer.
Keyword
MeSH Terms
-
Amino Acid Sequence
CD8-Positive T-Lymphocytes/immunology/metabolism
Epitopes/*immunology/therapeutic use
Female
*HLA-A Antigens
Human papillomavirus 16/*immunology
Humans
*Immunotherapy
Interferon-gamma/analysis/*biosynthesis
Leukocytes, Mononuclear/immunology/metabolism
T-Lymphocytes, Cytotoxic/immunology/metabolism
Uterine Cervical Neoplasms/*therapy
Epitopes
HLA-A Antigens
Interferon-gamma
Figure
Reference
-
1. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2:342–350.
Article2. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.
Article3. Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002; 55:244–265.
Article4. Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011; 29:4294–4301.
Article5. Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010; 10:845–852.
Article6. Centers for Disease Control and Prevention. National survey shows HPV vaccine rates trail other teen vaccines. 2011. accessed on 2011 August 25. Available at: https://www.cdc.gov/media/releases/2011/p0825_hpv_vaccine.htm.7. Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007; 298:743–753.
Article8. Cannistra SA, Niloff JM. Cancer of the uterine cervix. N Engl J Med. 1996; 334:1030–1038.
Article9. Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, et al. Immunotherapy for cervical cancer: research status and clinical potential. BioDrugs. 2010; 24:109–129.10. van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011; 23:252–257.
Article11. Jang S, Kim YT, Chung HW, Lee KR, Lim JB, Lee K. Identification of novel immunogenic human leukocyte antigen-A 2402-binding epitopes of human papillomavirus type 16 E7 for immunotherapy against human cervical cancer. Cancer. 2012; 118:2173–2183.
Article12. Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008; 26:5210–5215.
Article13. Ressing ME, Sette A, Brandt RM, Ruppert J, Wentworth PA, Hartman M, et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995; 154:5934–5943.14. Clayton J, Lonjou C. Allele and Haplotype frequencies for HLA loci in various ethnic groups. In : Charron D, editor. Genetic diversity of HLA. Functional and medical implications. Paris: EDK;1997. p. 665–820.15. Lim JB, Kim HO, Jeong SH, Ha JE, Jang S, Lee SG, et al. Identification of HLA-A*2402-restricted HCMV immediate early-1 (IE-1) epitopes as targets for CD8+ HCMV-specific cytotoxic T lymphocytes. J Transl Med. 2009; 7:72.
Article16. Rauser G, Einsele H, Sinzger C, Wernet D, Kuntz G, Assenmacher M, et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood. 2004; 103:3565–3572.
Article17. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article18. Schorge JO, Molpus KL, Koelliker D, Nikrui N, Goodman A, Fuller AF Jr. Stage IB and IIA cervical cancer with negative lymph nodes: the role of adjuvant radiotherapy after radical hysterectomy. Gynecol Oncol. 1997; 66:31–35.
Article19. Bonomi P, Blessing J, Ball H, Hanjani P, DiSaia PJ. A phase II evaluation of cisplatin and 5-fluorouracil in patients with advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989; 34:357–359.
Article20. Øvestad IT, Gudlaugsson E, Skaland I, Malpica A, Kruse AJ, Janssen EA, et al. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol. 2010; 23:1231–1240.
Article21. Jung AC, Guihard S, Krugell S, Ledrappier S, Brochot A, Dalstein V, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013; 132:E26–E36.
Article22. Kubo RT, Sette A, Grey HM, Appella E, Sakaguchi K, Zhu NZ, et al. Definition of specific peptide motifs for four major HLA-A alleles. J Immunol. 1994; 152:3913–3924.23. Jochmus I, Osen W, Altmann A, Buck G, Hofmann B, Schneider A, et al. Specificity of human cytotoxic T lymphocytes induced by a human papillomavirus type 16 E7-derived peptide. J Gen Virol. 1997; 78(Pt 7):1689–1695.
Article24. Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998; 4:2103–2109.25. Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines. 2016; 15:1327–1336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between Poor Immunogenicity of HLA-A2-Restricted Peptide Epitopes and Paucity of Naive CD8+ T-Cell Precursors in HLA-A2-Transgenic Mice
- Genetic Polymorphism in E7 Gene of Human Papillomavirus Type 16 Isolated from Uterine Cervical Cancer in Korean Women
- Analysis of human papillomavirus type 16 E6, E7 sequence variation in primary cervical cancer from Korean women and its relationship to the expression of immunomodulatory gene
- B Cells Transduced with HPV16 E6/E7-expressing Adenoviral Vector Can Efficiently Induce CTL-dependent Anti-Tumor Immunity
- Degradation of the Retinoblastoma Tumor Suppressor Protein by the Human Papillomavirus-16 E7 Variants