Yonsei Med J.
2017 Jan;58(1):35-42. 10.3349/ymj.2017.58.1.35.
Decitabine as a First-Line Treatment for Older Adults Newly Diagnosed with Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. jwcheong70@yuhs.ac
- KMID: 2374186
- DOI: http://doi.org/10.3349/ymj.2017.58.1.35
Abstract
- PURPOSE
Decitabine, a DNA hypomethylating agent, was recently approved for use in Korea for older adults with acute myeloid leukemia (AML) who are not candidates for standard chemotherapy. This study aimed to evaluate the role of decitabine as a first-line treatment for older adults with AML.
MATERIALS AND METHODS
Twenty-four patients with AML who received at least one course of decitabine (20 mg/m²/d intravenously for 5 days every 4 weeks) as a first-line therapy at Severance Hospital were evaluated retrospectively.
RESULTS
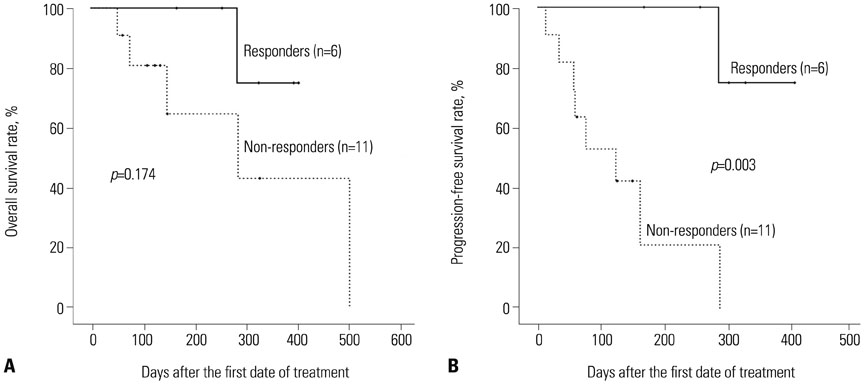
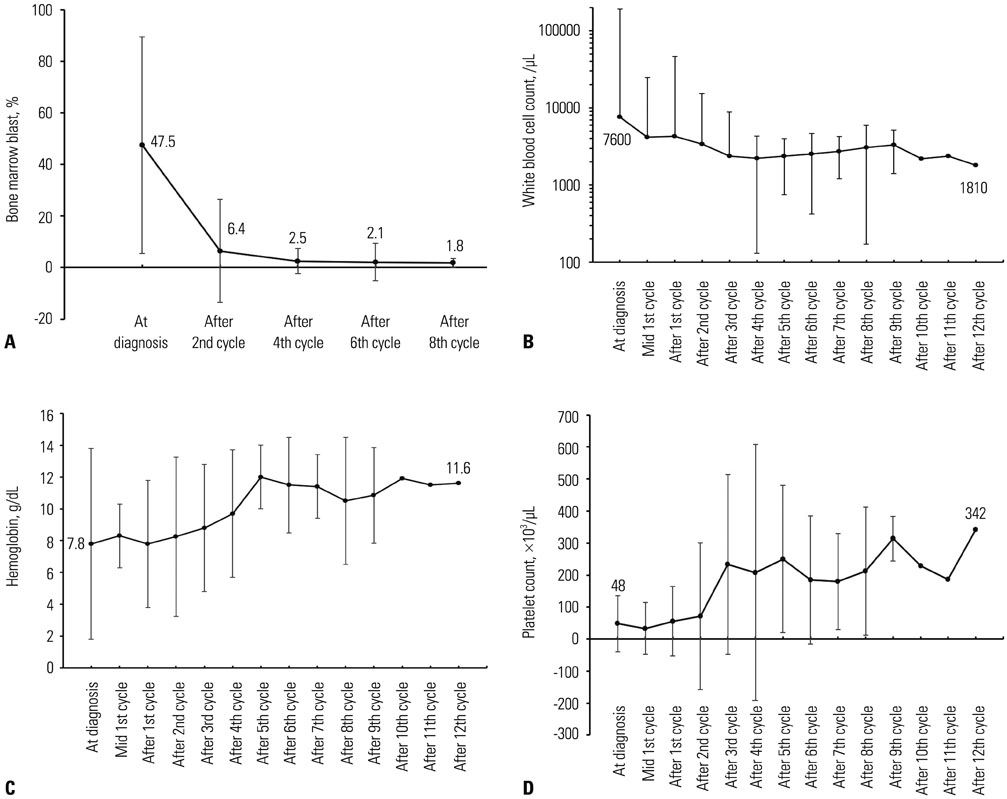
The median age of the patients was 73.5 years. The longest follow-up duration was 502 days. A total of 113 cycles of treatment were given to 24 patients, and the median number of cycles was four (range, 1-14). Thirteen patients dropped out because of death, no or loss of response, patient refusal, or transfer to another hospital. Twenty-one (87.5%) and 12 (50%) patients completed the second and fourth cycles, respectively, and responses to treatment were evaluated in 17. A complete response (CR) or CR with incomplete blood-count recovery was achieved in six (35.3%) patients, and the estimated median overall survival was 502 days. Ten patients developed grade >2 hematologic or non-hematologic toxicities. In univariate analysis, bone marrow blasts, lactate dehydrogenase, serum ferritin level, and bone marrow iron were significantly associated with response to decitabine.
CONCLUSION
Five-day decitabine treatment showed acceptable efficacy in older patients with AML who are unfit for conventional chemotherapy, with a CR rate 35.3% and about a median overall survival of 18 months.
Keyword
MeSH Terms
-
Aged
Antimetabolites, Antineoplastic/administration & dosage/*therapeutic use
Azacitidine/*analogs & derivatives/therapeutic use
DNA Methylation
Female
Humans
Leukemia, Myeloid, Acute/*drug therapy/mortality
Male
Middle Aged
Remission Induction
Republic of Korea
Retrospective Studies
Treatment Outcome
Antimetabolites, Antineoplastic
Azacitidine
Figure
Reference
-
1. Korea Central Cancer Registry NCC. Annual report of cancer statistics in Korea in 2012. Sejong: Ministry of Health and Welfare;2014.2. Sorm F, Veselý J. Effect of 5-aza-2'-deoxycytidine against leukemic and hemopoietic tissues in AKR mice. Neoplasma. 1968; 15:339–343.3. Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004; 103:1635–1640.
Article4. Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2'-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci U S A. 1994; 91:11797–11801.
Article5. Cruijsen M, Lübbert M, Wijermans P, Huls G. Clinical results of hypomethylating agents in AML treatment. J Clin Med. 2014; 4:1–17.
Article6. Claus R, Pfeifer D, Almstedt M, Zucknick M, Hackanson B, Plass C, et al. Decitabine induces very early in vivo DNA methylation changes in blasts from patients with acute myeloid leukemia. Leuk Res. 2013; 37:190–196.
Article7. Klco JM, Spencer DH, Lamprecht TL, Sarkaria SM, Wylie T, Magrini V, et al. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood. 2013; 121:1633–1643.
Article8. Fandy TE, Jiemjit A, Thakar M, Rhoden P, Suarez L, Gore SD. Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes. Clin Cancer Res. 2014; 20:1249–1258.
Article9. Shin DY, Park YS, Yang K, Kim GY, Kim WJ, Han MH, et al. Decitabine, a DNA methyltransferase inhibitor, induces apoptosis in human leukemia cells through intracellular reactive oxygen species generation. Int J Oncol. 2012; 41:910–918.
Article10. Cashen AF, Schiller GJ, O'Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010; 28:556–561.
Article11. Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006; 108:3271–3279.
Article12. Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012; 30:2670–2677.
Article13. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press;2008.14. Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003; 21:4642–4649.
Article15. Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001; 98:1312–1320.
Article16. Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood. 1998; 91:3607–3615.
Article17. Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B. N Engl J Med. 1995; 332:1671–1677.
Article18. Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011; 29:487–494.
Article19. Mayer J, Arthur C, Delaunay J, Mazur G, Thomas XG, Wierzbowska A, et al. Multivariate and subgroup analyses of a randomized, multinational, phase 3 trial of decitabine vs treatment choice of supportive care or cytarabine in older patients with newly diagnosed acute myeloid leukemia and poor- or intermediate-risk cytogenetics. BMC Cancer. 2014; 14:69.
Article20. Kadia TM, Thomas XG, Dmoszynska A, Wierzbowska A, Minden M, Arthur C, et al. Decitabine improves outcomes in older patients with acute myeloid leukemia and higher blast counts. Am J Hematol. 2015; 90:E139–E141.
Article21. Bhatnagar B, Duong VH, Gourdin TS, Tidwell ML, Chen C, Ning Y, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014; 55:1533–1537.
Article22. Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta. 2013; 1836:245–254.
Article23. Kikyo N, Hagiwara K, Yazaki Y, Okabe T. Growth stimulation of ferritin of human leukemia cells in vitro. J Cancer Res Clin Oncol. 1995; 121:76–78.
Article24. Armand P, Sainvil MM, Kim HT, Rhodes J, Cutler C, Ho VT, et al. Does iron overload really matter in stem cell transplantation? Am J Hematol. 2012; 87:569–572.
Article25. Bazuaye GN, Buser A, Gerull S, Tichelli A, Stern M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2012; 47:60–64.
Article26. Mahindra A, Bolwell B, Sobecks R, Rybicki L, Pohlman B, Dean R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009; 146:310–316.
Article27. Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010; 34:723–727.
Article28. Lebon D, Vergez F, Bertoli S, Harrivel V, De Botton S, Micol JB, et al. Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk Res. 2015; 39:818–821.
Article29. Kikuchi S, Kobune M, Iyama S, Sato T, Murase K, Kawano Y, et al. Prognostic significance of serum ferritin level at diagnosis in myelodysplastic syndrome. Int J Hematol. 2012; 95:527–534.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraparenchymal Myeloid Sarcoma and Subsequent Spinal Myeloid Sarcoma for Acute Myeloblastic Leukemia
- Long-term response in refractory AML following azacitidine-failed MDS by salvage decitabine-bridged allogenic transplantation
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- Current treatment for pediatric acute myeloid leukemia
- Myeloid Sarcoma of Peritoneum in Acute Myeloid Leukemia Patient with Inversion of Chromosome 16