Yonsei Med J.
2017 Jan;58(1):1-8. 10.3349/ymj.2017.58.1.1.
A Review of Modeling Approaches to Predict Drug Response in Clinical Oncology
- Affiliations
-
- 1Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea. kspark@yuhs.ac
- KMID: 2374182
- DOI: http://doi.org/10.3349/ymj.2017.58.1.1
Abstract
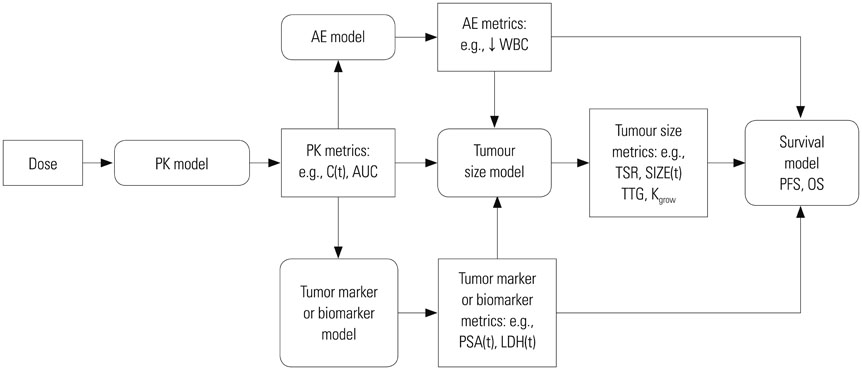
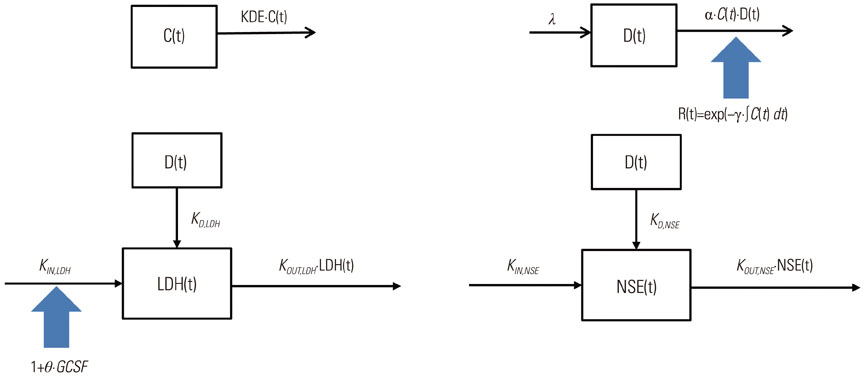
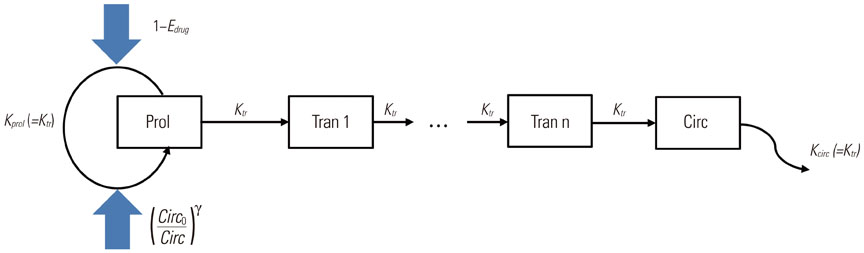
- Model-based approaches have emerged as important tools for quantitatively understanding temporal relationships between drug dose, concentration, and effect over the course of treatment, and have now become central to optimal drug development and tailored drug treatment. In oncology, the therapeutic index of a chemotherapeutic drug is typically narrow and a full dose-response relationship is not available, often because of treatment failure. Noting the benefits of model-based approaches and the low therapeutic index of oncology drugs, in recent years, modeling approaches have been increasingly used to streamline oncologic drug development through early identification and quantification of dose-response relationships. With this background, this report reviews publications that used model-based approaches to evaluate drug treatment outcome variables in oncology therapeutics, ranging from tumor size dynamics to tumor/biomarker time courses and survival response.
MeSH Terms
Figure
Reference
-
1. Sheiner LB, Rosenberg B, Melmon KL. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res. 1972; 5:411–459.
Article2. Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980; 8:553–571.
Article3. Stanski DR, Maitre PO. Population pharmacokinetics and pharmacodynamics of thiopental: the effect of age revisited. Anesthesiology. 1990; 72:412–422.4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article5. Giessen C, Laubender RP, Ankerst DP, Stintzing S, Modest DP, Mansmann U, et al. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: literature-based analysis from 50 randomized first-line trials. Clin Cancer Res. 2013; 19:225–235.
Article6. Sidhu R, Rong A, Dahlberg S. Evaluation of progression-free survival as a surrogate endpoint for survival in chemotherapy and targeted agent metastatic colorectal cancer trials. Clin Cancer Res. 2013; 19:969–976.
Article7. Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Lück HJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008; 26:1987–1992.
Article8. Sherrill B, Amonkar M, Wu Y, Hirst C, Stein S, Walker M, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008; 99:1572–1578.
Article9. Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004; 22:4442–4445.
Article10. Sharma MR, Maitland ML, Ratain MJ. RECIST: no longer the sharpest tool in the oncology clinical trials toolbox---point. Cancer Res. 2012; 72:5145–5149.
Article11. Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011; 17:907–917.
Article12. Ribba B, Holford NH, Magni P, Trocóniz I, Gueorguieva I, Girard P, et al. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacometrics Syst Pharmacol. 2014; 3:e113.
Article13. Bruno R, Claret L. On the use of change in tumor size to predict survival in clinical oncology studies: toward a new paradigm to design and evaluate phase II studies. Clin Pharmacol Ther. 2009; 86:136–138.
Article14. Claret L, Gupta M, Han K, Joshi A, Sarapa N, He J, et al. Evaluation of tumor-size response metrics to predict overall survival in Western and Chinese patients with first-line metastatic colorectal cancer. J Clin Oncol. 2013; 31:2110–2114.
Article15. Tham LS, Wang L, Soo RA, Lee SC, Lee HS, Yong WP, et al. A pharmacodynamic model for the time course of tumor shrinkage by gemcitabine + carboplatin in non-small cell lung cancer patients. Clin Cancer Res. 2008; 14:4213–4218.
Article16. Wang Y, Sung C, Dartois C, Ramchandani R, Booth BP, Rock E, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009; 86:167–174.
Article17. Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010; 66:357–371.
Article18. Stein A, Wang W, Carter AA, Chiparus O, Hollaender N, Kim H, et al. Dynamic tumor modeling of the dose-response relationship for everolimus in metastatic renal cell carcinoma using data from the phase 3 RECORD-1 trial. BMC Cancer. 2012; 12:311.
Article19. Maitland ML, Wu K, Sharma MR, Jin Y, Kang SP, Stadler WM, et al. Estimation of renal cell carcinoma treatment effects from disease progression modeling. Clin Pharmacol Ther. 2013; 93:345–351.
Article20. Bonate PL, Suttle AB. Modeling tumor growth kinetics after treatment with pazopanib or placebo in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2013; 72:231–240.
Article21. Claret L, Lu JF, Sun YN, Bruno R. Development of a modeling framework to simulate efficacy endpoints for motesanib in patients with thyroid cancer. Cancer Chemother Pharmacol. 2010; 66:1141–1149.
Article22. Frances N, Claret L, Bruno R, Iliadis A. Tumor growth modeling from clinical trials reveals synergistic anticancer effect of the capecitabine and docetaxel combination in metastatic breast cancer. Cancer Chemother Pharmacol. 2011; 68:1413–1419.
Article23. Hansson EK, Amantea MA, Westwood P, Milligan PA, Houk BE, French J, et al. PKPD Modeling of VEGF, sVEGFR-2, sVEGFR-3, and sKIT as predictors of tumor dynamics and overall survival following sunitinib treatment in GIST. CPT Pharmacometrics Syst Pharmacol. 2013; 11. 20. [Epub]. DOI: 10.1038/psp.2013.61.
Article24. Ribba B, Kaloshi G, Peyre M, Ricard D, Calvez V, Tod M, et al. A tumor growth inhibition model for low-grade glioma treated with chemotherapy or radiotherapy. Clin Cancer Res. 2012; 18:5071–5080.
Article25. Claret L, Girard P, Hoff PM, Van Cutsem E, Zuideveld KP, Jorga K, et al. Model-based prediction of phase III overall survival in colorectal cancer on the basis of phase II tumor dynamics. J Clin Oncol. 2009; 27:4103–4108.
Article26. Iliadis A, Barbolosi D. Optimizing drug regimens in cancer chemotherapy by an efficacy-toxicity mathematical model. Comput Biomed Res. 2000; 33:211–226.
Article27. Bender BC, Schindler E, Friberg LE. Population pharmacokinetic-pharmacodynamic modelling in oncology: a tool for predicting clinical response. Br J Clin Pharmacol. 2015; 79:56–71.
Article28. Korn RL, Crowley JJ. Overview: progression-free survival as an endpoint in clinical trials with solid tumors. Clin Cancer Res. 2013; 19:2607–2612.
Article29. You B, Girard P, Paparel P, Freyer G, Ruffion A, Charrié A, et al. Prognostic value of modeled PSA clearance on biochemical relapse free survival after radical prostatectomy. Prostate. 2009; 69:1325–1333.
Article30. You B, Fronton L, Boyle H, Droz JP, Girard P, Tranchand B, et al. Predictive value of modeled AUC(AFP-hCG), a dynamic kinetic parameter characterizing serum tumor marker decline in patients with nonseminomatous germ cell tumor. Urology. 2010; 76:423–429.e2.
Article31. Jonsson F, Claret L, Knight R, Olesnyckyj M, Jacques C, Rajkumar VS, et al. A longitudinal tumor growth inhibition model based on serum M-protein levels in patients with multiples myeloma treated by dexamethasone. Berlin: Population Approach Group in Europe (PAGE);2010.32. Desmée S, Mentré F, Veyrat-Follet C, Guedj J. Nonlinear mixed-effect models for prostate-specific antigen kinetics and link with survival in the context of metastatic prostate cancer: a comparison by simulation of two-stage and joint approaches. AAPS J. 2015; 17:691–699.
Article33. Kanefendt F, Lindauer A, Kinzig M, Scheulen M, Strumberg D, Fischer R, et al. Modeling sunitinib and biomarker response as potential predictors of time to progression in patients with metastatic colorectal cancer. Venice: Population Approach Group in Europe (PAGE);2012.34. Hansson EK. Pharmacometric Models for Biomarkers, Side Effects and Efficacy in Anticancer Drug Therapy. Uppsala: Acta Universitatis Upsaliensis;2012.35. Buil-Bruna N, López-Picazo JM, Moreno-Jiménez M, Martín-Algarra S, Ribba B, Trocóniz IF. A population pharmacodynamic model for lactate dehydrogenase and neuron specific enolase to predict tumor progression in small cell lung cancer patients. AAPS J. 2014; 16:609–619.
Article36. Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002; 20:4713–4721.
Article37. Agoram B, Heatherington AC, Gastonguay MR. Development and evaluation of a population pharmacokinetic-pharmacodynamic model of darbepoetin alfa in patients with nonmyeloid malignancies undergoing multicycle chemotherapy. AAPS J. 2006; 8:E552–E563.
Article38. Fetterly GJ, Owen JS, Stuyckens K, Passarell JA, Zannikos P, Soto-Matos A, et al. Semimechanistic pharmacokinetic/pharmacodynamic model for hepatoprotective effect of dexamethasone on transient transaminitis after trabectedin (ET-743) treatment. Cancer Chemother Pharmacol. 2008; 62:135–147.
Article39. Keizer RJ, Gupta A, Mac Gillavry MR, Jansen M, Wanders J, Beijnen JH, et al. A model of hypertension and proteinuria in cancer patients treated with the anti-angiogenic drug E7080. J Pharmacokinet Pharmacodyn. 2010; 37:347–363.
Article40. Hansson EK, Ma G, Amantea MA, French J, Milligan PA, Friberg LE, et al. PKPD modeling of predictors for adverse effects and overall survival in sunitinib-treated patients With GIST. CPT Pharmacometrics Syst Pharmacol. 2013; 2:e85.
Article41. Xie R, Mathijssen RH, Sparreboom A, Verweij J, Karlsson MO. Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther. 2002; 72:265–275.
Article42. Hénin E, You B, VanCutsem E, Hoff PM, Cassidy J, Twelves C, et al. A dynamic model of hand-and-foot syndrome in patients receiving capecitabine. Clin Pharmacol Ther. 2009; 85:418–425.
Article43. Holford N. A time to event tutorial for pharmacometricians. CPT Pharmacometrics Syst Pharmacol. 2013; 2:e43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Computational modeling of atrial fibrillation
- Pharmacokinetic and pharmacodynamic modeling in anesthetic field
- Comparative Study of First-in-Human Dose Estimation Approaches using Pharmacometrics
- Review of Statistical Methods for Evaluating the Performance of Survival or Other Time-to-Event Prediction Models (from Conventional to Deep Learning Approaches)
- Evolving role of modeling and simulation in drug development