Yonsei Med J.
2016 Sep;57(5):1054-1062. 10.3349/ymj.2016.57.5.1054.
Intraductal Carcinoma of the Prostate Gland: Recent Advances
- Affiliations
-
- 1Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Weill Medical College of Cornell University, Houston, TX, USA. jaero@houstonmethodist.org
- KMID: 2374148
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1054
Abstract
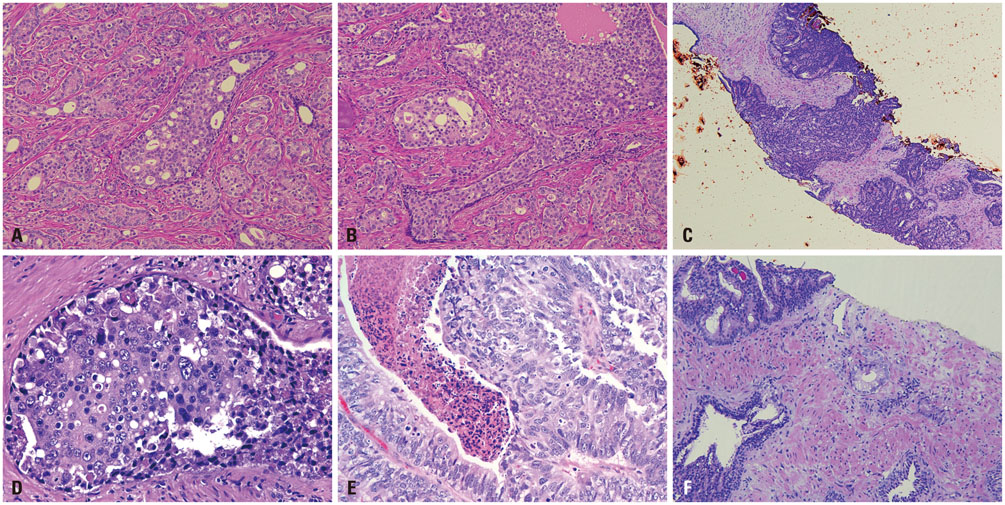
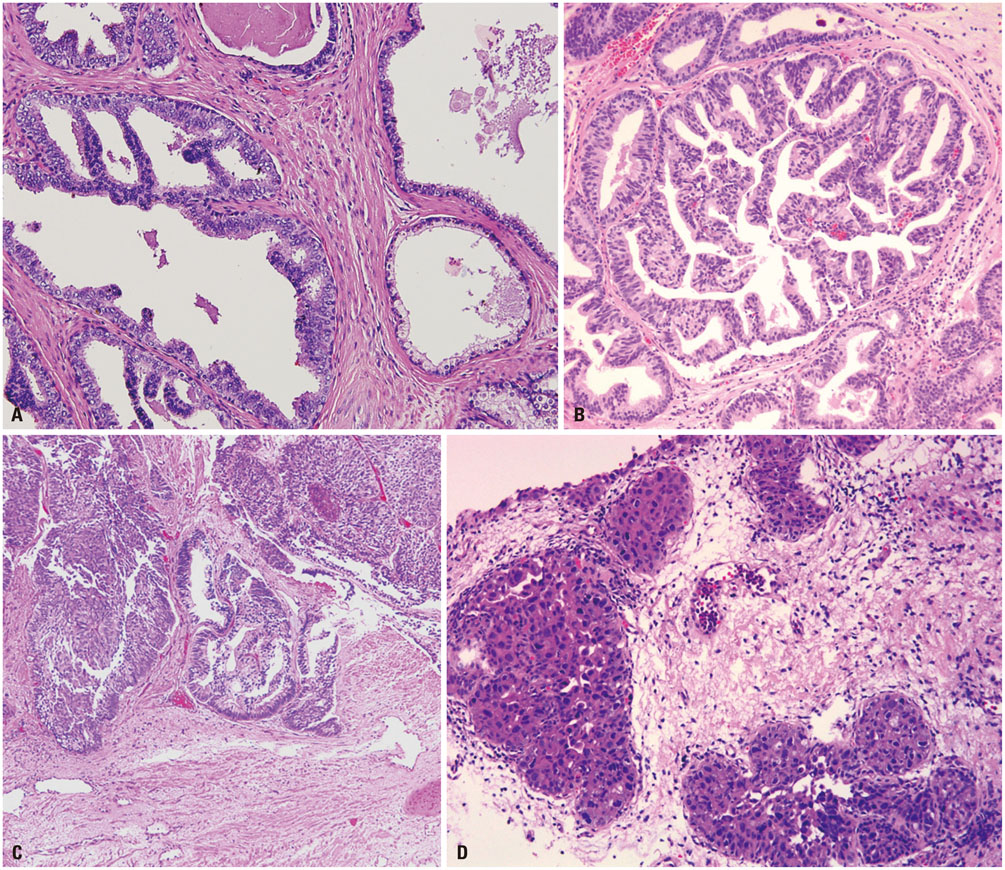
- Intraductal carcinoma of the prostate (IDC-P) is characterized by prostatic carcinoma involving ducts and/or acini. The presence of IDC-P is usually associated with a high-grade Gleason score, large tumor volume, and adverse prognostic parameters, including extraprostatic extension and seminal vesicle invasion. When present, IDC-P is associated with worse outcomes, regardless of treatment status. IDC-P is included in a broader diagnostic category of atypical cribriform lesions of the prostate gland. This category of lesions also includes high-grade prostatic intraepithelial neoplasia (HGPIN), urothelial carcinoma involving prostatic ducts or acini, and prostatic ductal adenocarcinoma, amongst other intraductal proliferations. Differentiating between these entities is important as they have differing therapeutic and prognostic implications for patients, although differential diagnosis thereof is not always straightforward. The present review discusses IDC-P in regards to its morphological characteristics, molecular features, and clinical outcomes. Given the current state of knowledge, the presence of IDC-P should be evaluated and documented correctly in both radical prostatectomy and needle biopsy specimens, and the clinical implications thereof should be taken into consideration during treatment and follow up.
Keyword
MeSH Terms
-
Carcinoma, Acinar Cell/chemistry/*diagnosis/pathology
Carcinoma, Ductal/chemistry/*diagnosis/pathology
Carcinoma, Transitional Cell/chemistry/*diagnosis/pathology
Diagnosis, Differential
Humans
Male
Neoplasm Grading
Prostatic Intraepithelial Neoplasia/chemistry/*diagnosis/pathology
Prostatic Neoplasms/chemically induced/*diagnosis/*pathology
Tumor Burden
Figure
Reference
-
1. Rhamy RK, Buchanan RD, Spalding MJ. Intraductal carcinoma of the prostate gland. J Urol. 1973; 109:457–460.
Article2. Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol. 2010; 184:1328–1333.
Article3. Epstein JI, Netto GJ. Biopsy Interpretation of the Prostate. 5th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins;2015.4. Zhou M. Intraductal carcinoma of the prostate: the whole story. Pathology. 2013; 45:533–539.
Article5. Catalona WJ, Kadmon D, Martin SA. Surgical considerations in treatment of intraductal carcinoma of the prostate. J Urol. 1978; 120:259–261.
Article6. Kovi J, Jackson MA, Heshmat MY. Ductal spread in prostatic carcinoma. Cancer. 1985; 56:1566–1573.
Article7. McNeal JE, Reese JH, Redwine EA, Freiha FS, Stamey TA. Cribriform adenocarcinoma of the prostate. Cancer. 1986; 58:1714–1719.
Article8. Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987; 59:788–794.
Article9. Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod Pathol. 2006; 19:1528–1535.
Article10. Cohen RJ, Wheeler TM, Bonkhoff H, Rubin MA. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med. 2007; 131:1103–1109.
Article11. Shah RB, Magi-Galluzzi C, Han B, Zhou M. Atypical cribriform lesions of the prostate: relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am J Surg Pathol. 2010; 34:470–477.
Article12. Cohen RJ, McNeal JE, Baillie T. Patterns of differentiation and proliferation in intraductal carcinoma of the prostate: significance for cancer progression. Prostate. 2000; 43:11–19.
Article13. Van der Kwast T, Al Daoud N, Collette L, Sykes J, Thoms J, Milosevic M, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer. 2012; 48:1318–1325.
Article14. Cohen RJ, Chan WC, Edgar SG, Robinson E, Dodd N, Hoscek S, et al. Prediction of pathological stage and clinical outcome in prostate cancer: an improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br J Urol. 1998; 81:413–418.
Article15. Watts K, Li J, Magi-Galluzzi C, Zhou M. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology. 2013; 63:574–579.
Article16. Rubin MA, de La Taille A, Bagiella E, Olsson CA, O'Toole KM. Cribriform carcinoma of the prostate and cribriform prostatic intraepithelial neoplasia: incidence and clinical implications. Am J Surg Pathol. 1998; 22:840–848.
Article17. Dawkins HJ, Sellner LN, Turbett GR, Thompson CA, Redmond SL, McNeal JE, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate. 2000; 44:265–270.
Article18. McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol. 1996; 20:802–814.19. Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol. 1998; 29:1119–1123.
Article20. O'Brien BA, Cohen RJ, Wheeler TM, Moorin RE. A post-radical-prostatectomy nomogram incorporating new pathological variables and interaction terms for improved prognosis. BJU Int. 2011; 107:389–395.21. Kimura K, Tsuzuki T, Kato M, Saito AM, Sassa N, Ishida R, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate. 2014; 74:680–687.
Article22. Lotan TL, Gumuskaya B, Rahimi H, Hicks JL, Iwata T, Robinson BD, et al. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol. 2013; 26:587–603.
Article23. Bettendorf O, Schmidt H, Staebler A, Grobholz R, Heinecke A, Boecker W, et al. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer. 2008; 47:565–572.
Article24. Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005; 310:644–648.
Article25. Han B, Suleman K, Wang L, Siddiqui J, Sercia L, Magi-Galluzzi C, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: Implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol. 2010; 34:478–485.26. Morais CL, Han JS, Gordetsky J, Nagar MS, Anderson AE, Lee S, et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am J Surg Pathol. 2015; 39:169–178.
Article27. Tomlins SA, Palanisamy N, Siddiqui J, Chinnaiyan AM, Kunju LP. Antibody-based detection of ERG rearrangements in prostate core biopsies, including diagnostically challenging cases: ERG staining in prostate core biopsies. Arch Pathol Lab Med. 2012; 136:935–946.
Article28. Miyai K, Divatia MK, Shen SS, Miles BJ, Ayala AG, Ro JY. Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: a comparison between "precursor-like" and "regular type" lesions. Int J Clin Exp Pathol. 2014; 7:2518–2526.29. Khani F, Epstein JI. Prostate biopsy specimens with Gleason 3+3=6 and intraductal carcinoma: radical prostatectomy findings and clinical outcomes. Am J Surg Pathol. 2015; 39:1383–1389.30. Bostwick DG, Amin MB, Dundore P, Marsh W, Schultz DS. Architectural patterns of high-grade prostatic intraepithelial neoplasia. Hum Pathol. 1993; 24:298–310.
Article31. Amin MB, Schultz DS, Zarbo RJ. Analysis of cribriform morphology in prostatic neoplasia using antibody to high-molecular-weight cytokeratins. Arch Pathol Lab Med. 1994; 118:260–264.32. Miyai K, Divatia MK, Shen SS, Miles BJ, Ayala AG, Ro JY. Clinicopathological analysis of intraductal proliferative lesions of prostate: intraductal carcinoma of prostate, high-grade prostatic intraepithelial neoplasia, and atypical cribriform lesion. Hum Pathol. 2014; 45:1572–1581.
Article33. Seipel AH, Wiklund F, Wiklund NP, Egevad L. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Arch. 2013; 462:429–436.
Article34. Samaratunga H, Duffy D, Yaxley J, Delahunt B. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol. 2010; 41:281–285.
Article35. Aydin H, Zhang J, Samaratunga H, Tan N, Magi-Galluzzi C, Klein E, et al. Ductal adenocarcinoma of the prostate diagnosed on transurethral biopsy or resection is not always indicative of aggressive disease: implications for clinical management. BJU Int. 2010; 105:476–480.
Article36. Herawi M, Epstein JI. Immunohistochemical antibody cocktail staining (p63/HMWCK/AMACR) of ductal adenocarcinoma and Gleason pattern 4 cribriform and noncribriform acinar adenocarcinomas of the prostate. Am J Surg Pathol. 2007; 31:889–894.
Article37. Christensen WN, Steinberg G, Walsh PC, Epstein JI. Prostatic duct adenocarcinoma. Findings at radical prostatectomy. Cancer. 1991; 67:2118–2124.
Article38. Ro JY, Ayala AG, Wishnow KI, Ordóñez NG. Prostatic duct adenocarcinoma with endometrioid features: immunohistochemical and electron microscopic study. Semin Diagn Pathol. 1988; 5:301–311.39. Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features. Clinical, pathologic, and ultrastructural findings. Am J Surg Pathol. 1985; 9:595–609.40. Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer. 1986; 57:111–119.
Article41. Brinker DA, Potter SR, Epstein JI. Ductal adenocarcinoma of the prostate diagnosed on needle biopsy: correlation with clinical and radical prostatectomy findings and progression. Am J Surg Pathol. 1999; 23:1471–1479.42. Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol. 2010; 184:2303–2307.
Article43. Amin A, Epstein JI. Pathologic stage of prostatic ductal adenocarcinoma at radical prostatectomy: effect of percentage of the ductal component and associated grade of acinar adenocarcinoma. Am J Surg Pathol. 2011; 35:615–619.
Article44. Meeks JJ, Zhao LC, Cashy J, Kundu S. Incidence and outcomes of ductal carcinoma of the prostate in the USA: analysis of data from the Surveillance, Epidemiology, and End Results program. BJU Int. 2012; 109:831–834.
Article45. Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasialike ductal adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol. 2008; 32:1060–1067.
Article46. Hameed O, Humphrey PA. Stratified epithelium in prostatic adenocarcinoma: a mimic of high-grade prostatic intraepithelial neoplasia. Mod Pathol. 2006; 19:899–906.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Intraductal Papilloma arising in the Parotid Gland
- Intraductal Carcinoma of Prostate: A Comprehensive and Concise Review
- Adenoid cystic carcinoma of the prostate gland: pathological review with a case report
- A Case of Intraductal Papilloma in the Parotid Gland
- A Case of Lymphoepithelioma-Like Carcinoma in the Thyroid Gland