Yonsei Med J.
2016 May;57(3):652-657. 10.3349/ymj.2016.57.3.652.
Comparative Analysis of Liver Injury-Associated Cytokines in Acute Hepatitis A and B
- Affiliations
-
- 1Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea. ecshin@kaist.ac.kr
- 2Department of Infectious Diseases, International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, College of Medicine, Seoul National University, Seongnam, Korea.
- 4Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea.
- KMID: 2374085
- DOI: http://doi.org/10.3349/ymj.2016.57.3.652
Abstract
- PURPOSE
Acute hepatitis A (AHA) and acute hepatitis B (AHB) are caused by an acute infection of the hepatitis A virus and the hepatitis B virus, respectively. In both AHA and AHB, liver injury is known to be mediated by immune cells and cytokines. In this study, we measured serum levels of various cytokines and T-cell cytotoxic proteins in patients with AHA or AHB to identify liver injury-associated cytokines.
MATERIALS AND METHODS
Forty-six patients with AHA, 16 patients with AHB, and 14 healthy adults were enrolled in the study. Serum levels of 17 cytokines and T-cell cytotoxic proteins were measured by enzyme-linked immunosorbent assays or cytometric bead arrays and analyzed for correlation with serum alanine aminotransferase (ALT) levels.
RESULTS
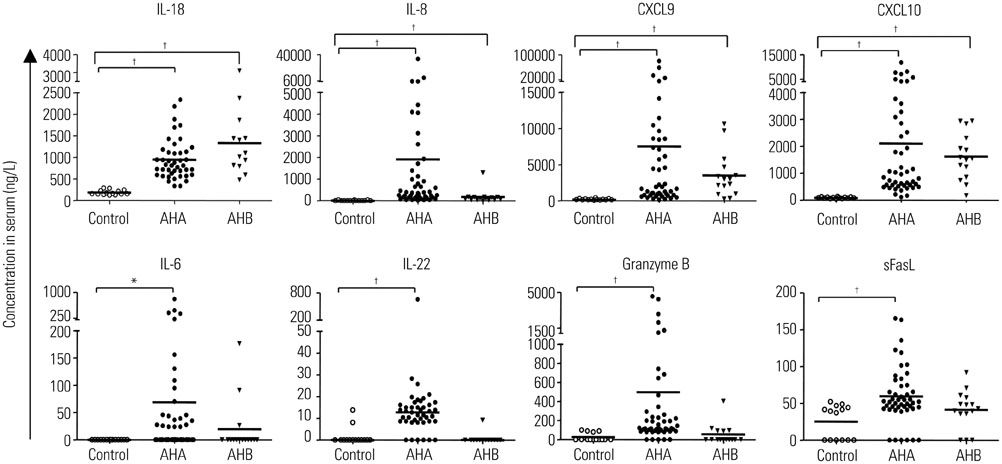
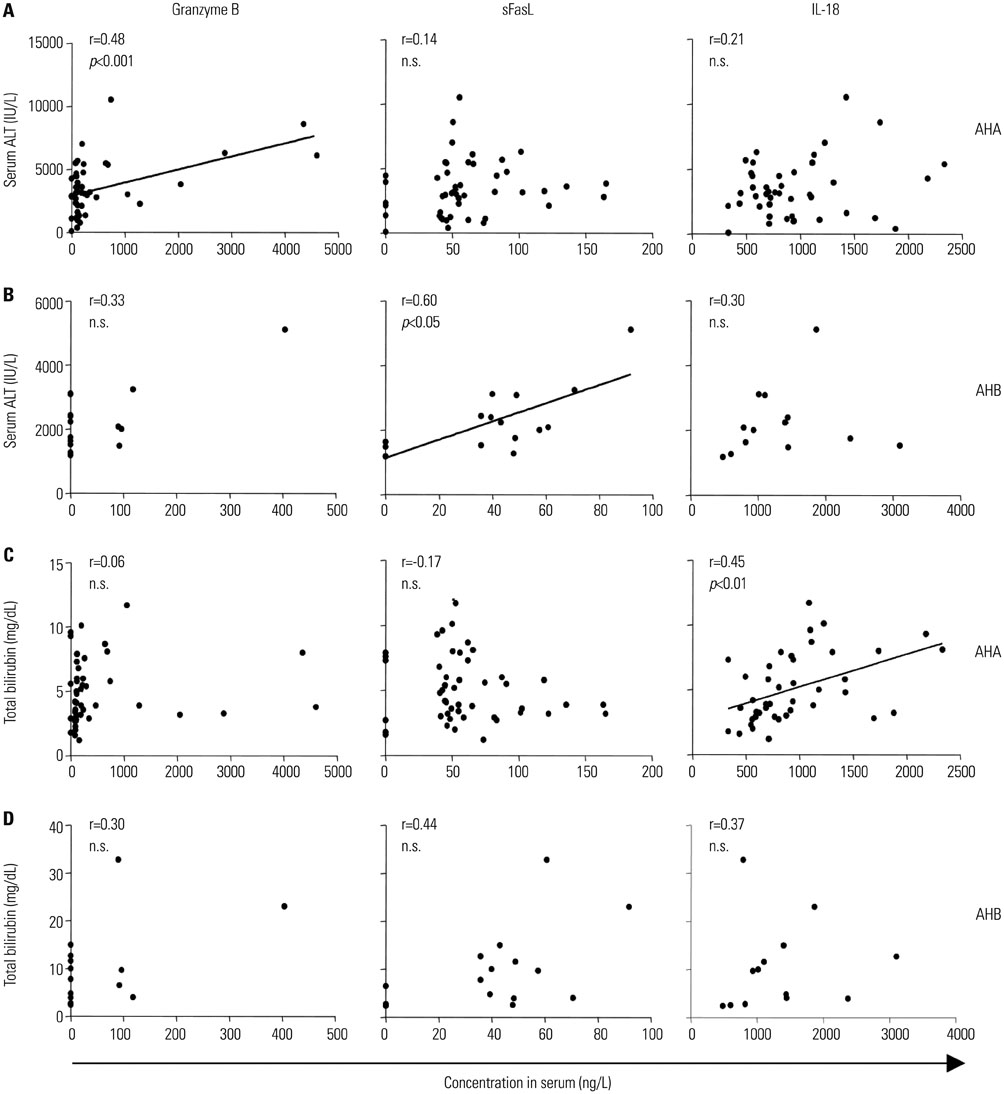
Interleukin (IL)-18, IL-8, CXCL9, and CXCL10 were significantly elevated in both AHA and AHB. IL-6, IL-22, granzyme B, and soluble Fas ligand (sFasL) were elevated in AHA but not in AHB. In both AHA and AHB, the serum level of CXCL10 significantly correlated with the peak ALT level. Additionally, the serum level of granzyme B in AHA and the serum level of sFasL in AHB correlated with the peak ALT level.
CONCLUSION
We identified cytokines and T-cell cytotoxic proteins associated with liver injury in AHA and AHB. These findings deepen the existing understanding of immunological mechanisms responsible for liver injury in acute viral hepatitis.
MeSH Terms
-
Acute Disease
Adult
Alanine Transaminase/blood
Biomarkers/blood
Cytokines/*blood
Enzyme-Linked Immunosorbent Assay
Fas Ligand Protein/blood
Female
Hepatitis A/blood/virology
Hepatitis A virus/*genetics/immunology
Hepatitis B/blood/virology
Hepatitis B virus/*genetics/immunology
Humans
Interleukin-6/blood
Interleukin-8/blood
Interleukins/blood
Liver Failure/immunology/metabolism/*pathology
Male
Middle Aged
T-Lymphocytes, Cytotoxic/immunology/*metabolism
Alanine Transaminase
Biomarkers
Cytokines
Fas Ligand Protein
Interleukin-6
Interleukin-8
Interleukins
Figure
Reference
-
1. Chung SJ, Kim TY, Kim SM, Roh M, Yu MY, Lee JH, et al. Changes in the seroprevalence of IgG anti-hepatitis A virus between 2001 and 2013: experience at a single center in Korea. Clin Mol Hepatol. 2014; 20:162–167.
Article2. Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006; 28:101–111.
Article3. Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. 2012; 4:68–73.
Article4. Collier MG, Tong X, Xu F. Hepatitis A hospitalizations in the United States, 2002-2011. Hepatology. 2015; 61:481–485.
Article5. Koff RS. Hepatitis A. Lancet. 1998; 351:1643–1649.
Article6. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015; 479-480:672–686.
Article7. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380:2095–2128.8. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014; 384:2053–2063.
Article9. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005; 5:215–229.
Article10. Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006; 1:23–61.
Article11. Walker CM, Feng Z, Lemon SM. Reassessing immune control of hepatitis A virus. Curr Opin Virol. 2015; 11:7–13.
Article12. Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome. Nat Rev Immunol. 2010; 10:514–526.
Article13. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007; 25:821–852.
Article14. Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007; 25:171–192.
Article15. Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007; 25:787–820.16. Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014; 147:577–594.e1.
Article17. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011; 89:207–215.
Article18. Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004; 39:1332–1342.
Article19. Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology. 2011; 54:252–261.
Article20. Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang JX, et al. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011; 56:174–179.
Article21. Fadel SA, Bromley SK, Medoff BD, Luster AD. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur J Immunol. 2008; 38:3376–3387.
Article22. Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, et al. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006; 177:1855–1863.
Article23. Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005; 79:11457–11466.
Article24. Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, et al. Delayed induction, not impaired recruitment, of specific CD8+ T cells causes the late onset of acute hepatitis C. Gastroenterology. 2011; 141:686–695.
Article25. Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011; 52:871–878.
Article26. Grebely J, Feld JJ, Applegate T, Matthews GV, Hellard M, Sherker A, et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology. 2013; 57:2124–2134.
Article27. Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003; 74:360–369.
Article28. Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004; 39:1220–1229.
Article29. Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008; 48:1440–1450.
Article30. Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002; 97:2861–2870.
Article31. Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010; 52:330–339.
Article32. Shiota G, Oyama K, Noguchi N, Takano Y, Kitaoka S, Kawasaki H. Clinical significance of serum soluble Fas ligand in patients with acute self-limited and fulminant hepatitis. Res Commun Mol Pathol Pharmacol. 1998; 101:3–12.33. Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009; 6:15–25.
Article34. Duffy D, Mamdouh R, Laird M, Soneson C, Le Fouler L, El-Daly M, et al. The ABCs of viral hepatitis that define biomarker signatures of acute viral hepatitis. Hepatology. 2014; 59:1273–1282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathological Findings of a Renal Biopsy Showing Chronic Hepatitis B in A Patient with Acute Hepatitis A
- Cytokines in Chronic Hepatitis B and C Virus Infections
- A Case of Acute Renal Failure Associated with Non-fulminant Acute Hepatitis A
- Clinical Implications of Chemokines in Acute and Chronic Hepatitis C Virus Infection
- Viral Hepatitis: Focus on Clinical Manifestations of Hepatitis A, B and C