Yonsei Med J.
2016 Mar;57(2):373-381. 10.3349/ymj.2016.57.2.373.
Anti-Proliferative Effects of Rutin on OLETF Rat Vascular Smooth Muscle Cells Stimulated by Glucose Variability
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Hallym University College of Medicine, Seoul, Korea. hjoonyoo@gmail.com
- 2Department of Internal Medicine, National Medical Center, Seoul, Korea.
- KMID: 2374042
- DOI: http://doi.org/10.3349/ymj.2016.57.2.373
Abstract
- PURPOSE
Proliferation of vascular smooth muscle cells (VSMCs) plays a crucial role in atherosclerosis. Rutin is a major representative of the flavonol subclass of flavonoids and has various pharmacological activities. Currently, data are lacking regarding its effects on VSMC proliferation induced by intermittent hyperglycemia. Here, we demonstrate the effects of rutin on VSMC proliferation and migration according to fluctuating glucose levels.
MATERIALS AND METHODS
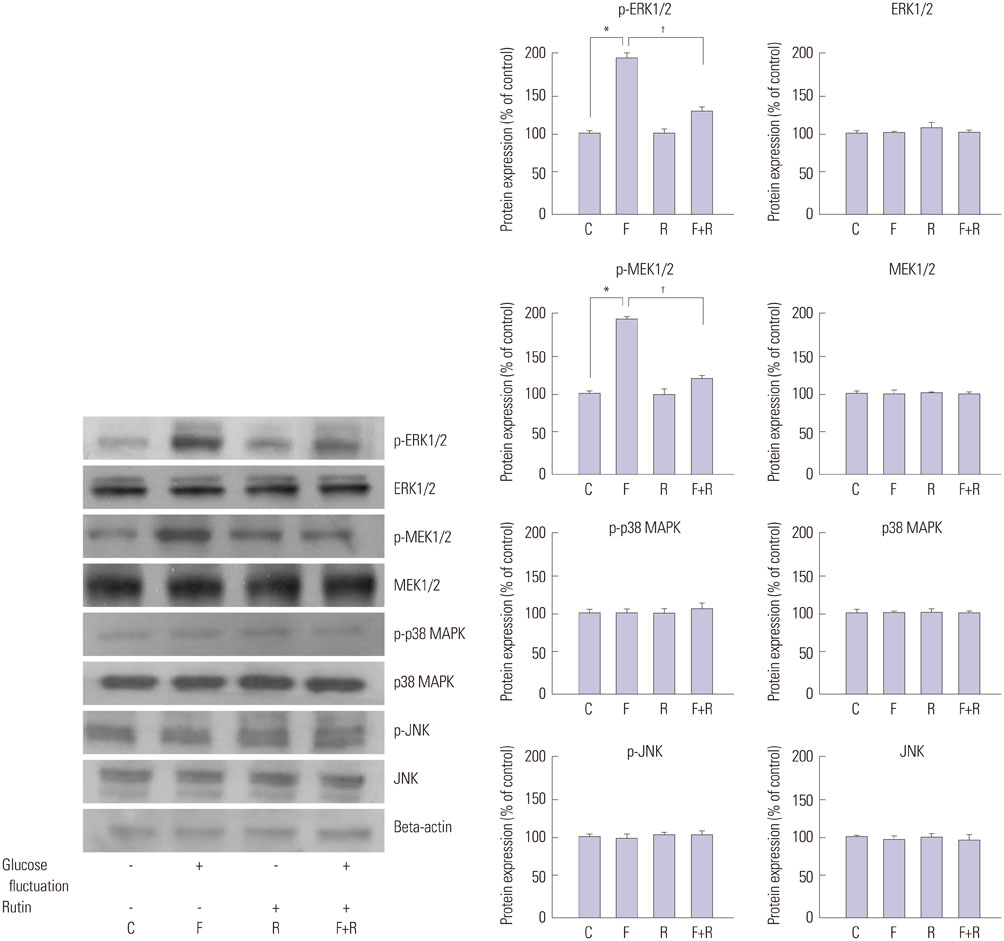
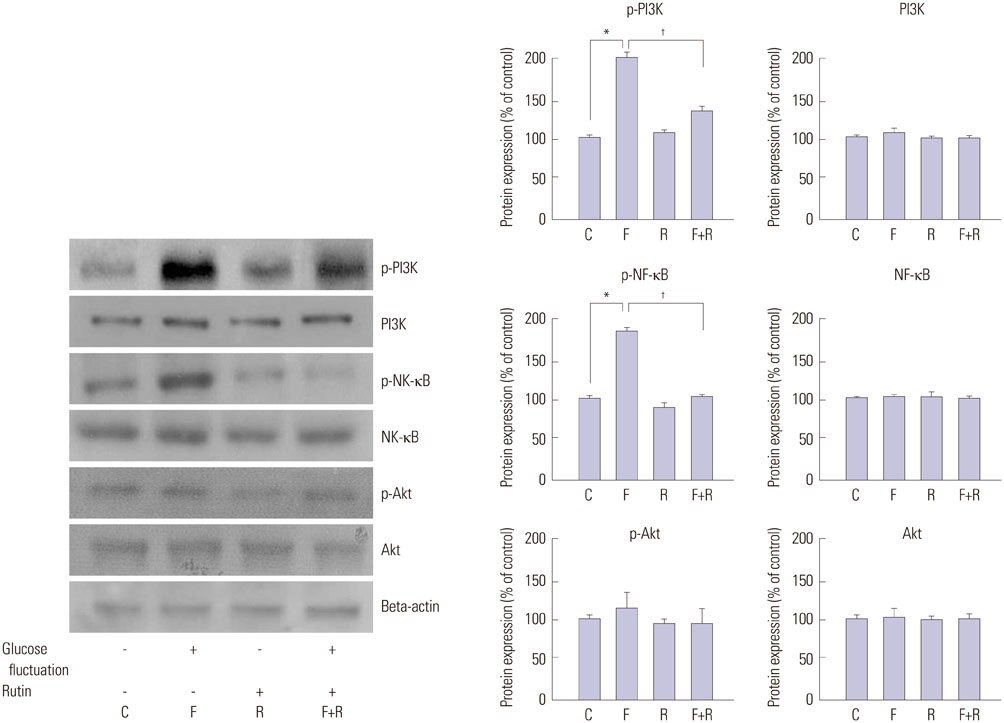
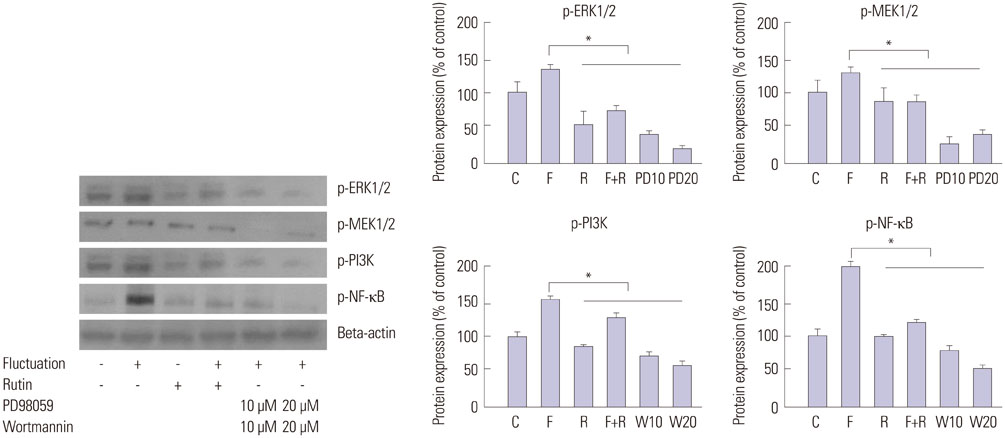
Primary cultures of male Otsuka Long-Evans Tokushima Fatty (OLETF) rat VSMCs were obtained from enzymatically dissociated rat thoracic aortas. VSMCs were incubated for 72 h with alternating normal (5.5 mmol/L) and high (25.0 mmol/L) glucose media every 12 h. Proliferation and migration of VSMCs, the proliferative molecular pathway [including p44/42 mitogen-activated protein kinases (MAPK), mitogen-activated protein kinase kinase 1/2 (MEK1/2), p38 MAPK, phosphoinositide 3-kinase (PI3K), c-Jun N-terminal protein kinase (JNK), nuclear factor kappa B (NF-kappaB), and Akt], the migratory pathway (big MAPK 1, BMK1), reactive oxygen species (ROS), and apoptotic pathway were analyzed.
RESULTS
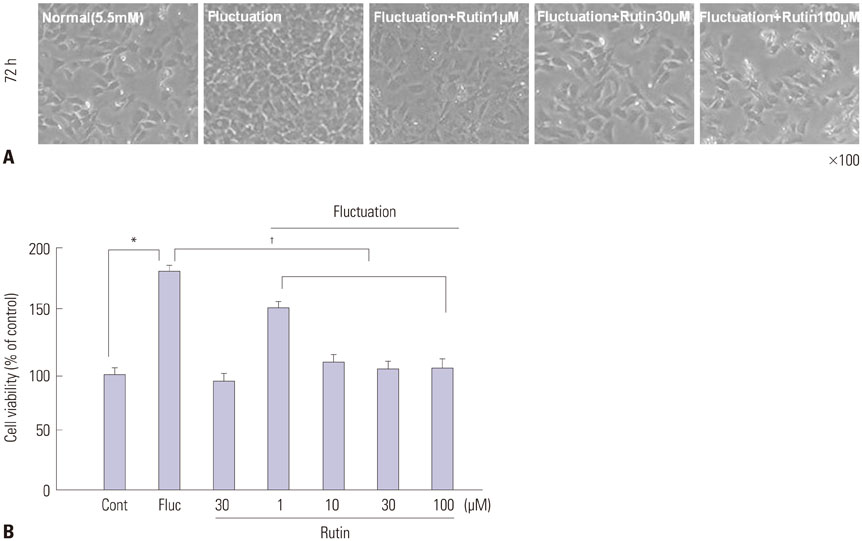
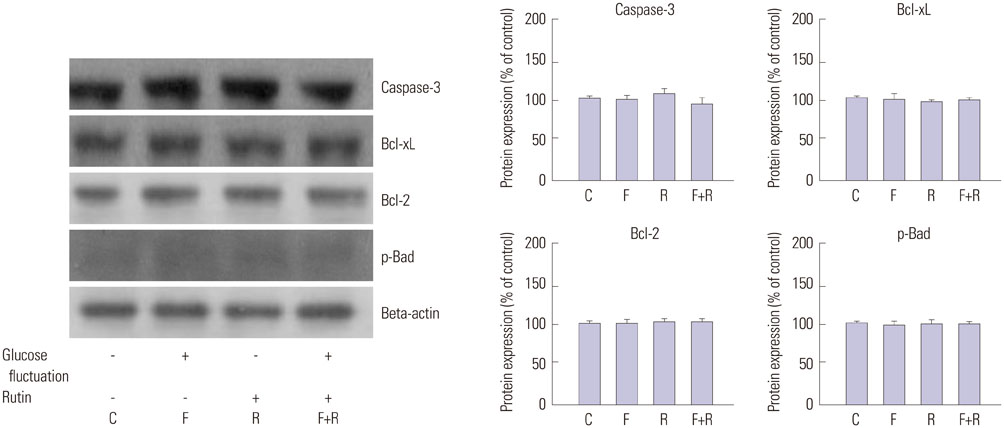
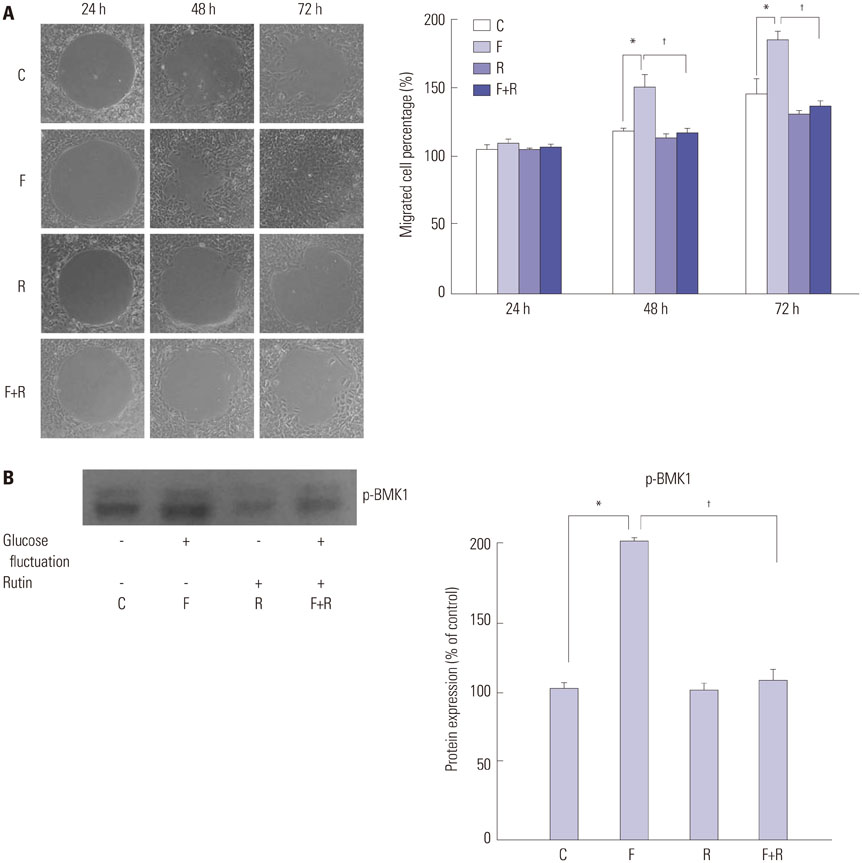
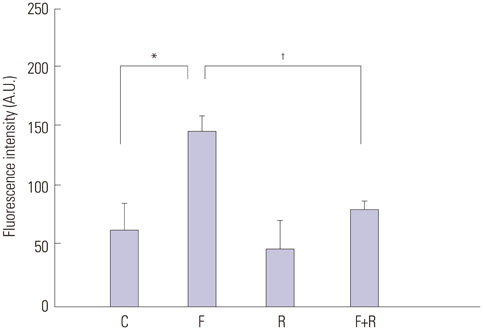
We found enhanced proliferation and migration of VSMCs when cells were incubated in intermittent high glucose conditions, compared to normal glucose. These effects were lowered upon rutin treatment. Intermittent treatment with high glucose for 72 h increased the expression of phospho-p44/42 MAPK (extracellular signal regulated kinase 1/2, ERK1/2), phospho-MEK1/2, phospho-PI3K, phospho-NF-kappaB, phospho-BMK1, and ROS, compared to treatment with normal glucose. These effects were suppressed by rutin. Phospho-p38 MAPK, phospho-Akt, JNK, and apoptotic pathways [B-cell lymphoma (Bcl)-xL, Bcl-2, phospho-Bad, and caspase-3] were not affected by fluctuations in glucose levels.
CONCLUSION
Fluctuating glucose levels increased proliferation and migration of OLETF rat VSMCs via MAPK (ERK1/2), BMK1, PI3K, and NF-kappaB pathways. These effects were inhibited by the antioxidant rutin.
Keyword
MeSH Terms
-
Animals
Caspase 3/metabolism
Cell Movement/*drug effects
Cell Proliferation/*drug effects
Flavonoids/*pharmacology
Glucose/*metabolism/pharmacology
JNK Mitogen-Activated Protein Kinases
MAP Kinase Kinase 1
Male
Mitogen-Activated Protein Kinase 3
Muscle, Smooth, Vascular/cytology/*drug effects/enzymology
Myocytes, Smooth Muscle/metabolism
NF-kappa B/metabolism
Phosphatidylinositol 3-Kinases
Protein Kinase Inhibitors/*pharmacology
Rats
Rats, Inbred OLETF
Rats, Long-Evans
Reactive Oxygen Species/metabolism
Rutin/*pharmacology
p38 Mitogen-Activated Protein Kinases/metabolism
Caspase 3
Glucose
Flavonoids
JNK Mitogen-Activated Protein Kinases
NF-kappa B
MAP Kinase Kinase 1
Mitogen-Activated Protein Kinase 3
Phosphatidylinositol 3-Kinases
Protein Kinase Inhibitors
Reactive Oxygen Species
Rutin
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Singh A, Donnino R, Weintraub H, Schwartzbard A. Effect of strict glycemic control in patients with diabetes mellitus on frequency of macrovascular events. Am J Cardiol. 2013; 112:1033–1038.
Article2. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
Article3. Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986; 58:427–444.
Article4. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001; 44:2107–2114.
Article5. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–1687.
Article6. Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, et al. Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vascul Pharmacol. 2010; 53:273–280.
Article7. Park S, Lim S, Chang W, Song H, Lee S, Song BW, et al. The inhibition of insulin-stimulated proliferation of vascular smooth muscle cells by rosiglitazone is mediated by the Akt-mTOR-P70S6K pathway. Yonsei Med J. 2008; 49:592–600.
Article8. Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999; 48:855–864.
Article9. Yoshizumi M, Kyotani Y, Zhao J, Nagayama K, Ito S, Tsuji Y, et al. Role of big mitogen-activated protein kinase 1 (BMK1) / extracellular signal-regulated kinase 5 (ERK5) in the pathogenesis and progression of atherosclerosis. J Pharmacol Sci. 2012; 120:259–263.
Article10. Yoo HJ, Kozaki K, Akishita M, Watanabe M, Eto M, Nagano K, et al. Augmented Ca2+ influx is involved in the mechanism of enhanced proliferation of cultured vascular smooth muscle cells from spontaneously diabetic Goto-Kakizaki rats. Atherosclerosis. 1997; 131:167–175.
Article11. Bochaton-Piallat ML, Ropraz P, Gabbiani F, Gabbiani G. Phenotypic heterogeneity of rat arterial smooth muscle cell clones. Implications for the development of experimental intimal thickening. Arterioscler Thromb Vasc Biol. 1996; 16:815–820.12. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006; 545:51–64.
Article13. Belcaro G, Cesarone MR, Ledda A, Cacchio M, Ruffini I, Ricci A, et al. 5-Year control and treatment of edema and increased capillary filtration in venous hypertension and diabetic microangiopathy using O-(beta-hydroxyethyl)-rutosides: a prospective comparative clinical registry. Angiology. 2008; 59:Suppl 1. 14S–20S.14. Rodrigues AM, Marcilio Fdos S, Frazão Muzitano M, Giraldi-Guimarães A. Therapeutic potential of treatment with the flavonoid rutin after cortical focal ischemia in rats. Brain Res. 2013; 1503:53–61.
Article15. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011; 82:513–523.
Article16. Araújo JR, Gonçalves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011; 31:77–87.
Article17. Kulisić T, Krisko A, Dragović-Uzelac V, Milos M, Pifat G. The effects of essential oils and aqueous tea infusions of oregano (Origanum vulgare L. spp. hirtum), thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) on the copper-induced oxidation of human low-density lipoproteins. Int J Food Sci Nutr. 2007; 58:87–93.18. Hsu IP, Jou HJ, Huang CW, Wang TA, Wu WH. The effects of soygerm extracts on blood lipoproteins, antioxidative capacity and urinary estrogen metabolites in postmenopausal women on hormone therapy. Int J Gynaecol Obstet. 2007; 98:29–33.
Article19. Botelho FV, Alvarez-Leite JI, Lemos VS, Pimenta AM, Calado HD, Matencio T, et al. Physicochemical study of floranol, its copper(II) and iron(III) complexes, and their inhibitory effect on LDL oxidation. J Inorg Biochem. 2007; 101:935–943.
Article20. Milde J, Elstner EF, Grassmann J. Synergistic effects of phenolics and carotenoids on human low-density lipoprotein oxidation. Mol Nutr Food Res. 2007; 51:956–961.
Article21. Alipui C, Ramos K, Tenner TE Jr. Alterations of rabbit aortic smooth muscle cell proliferation in diabetes mellitus. Cardiovasc Res. 1993; 27:1229–1232.
Article22. Natarajan R, Gonzales N, Xu L, Nadler JL. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem Biophys Res Commun. 1992; 187:552–560.
Article23. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287:2570–2581.24. Natali A, Vichi S, Landi P, Severi S, L'Abbate A, Ferrannini E. Coronary atherosclerosis in Type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000; 43:632–641.
Article25. Wang C, Lv L, Yang Y, Chen D, Liu G, Chen L, et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2012; 76:810–815.
Article26. Kim M, Chung H, Yoon C, Lee E, Kim T, Kim T, et al. Increase of INS-1 cell apoptosis under glucose fluctuation and the involvement of FOXO-SIRT pathway. Diabetes Res Clin Pract. 2012; 98:132–139.
Article27. Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest. 2012; 122:2104–2113.
Article28. Lee WH, Ku SK, Bae JS. Barrier protective effects of rutin in LPSinduced inflammation in vitro and in vivo. Food Chem Toxicol. 2012; 50:3048–3055.
Article29. Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS, Kim HJ, et al. Rutin from Lonicera japonica inhibits myocardial ischemia/reperfusion-induced apoptosis in vivo and protects H9c2 cells against hydrogen peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro. Food Chem Toxicol. 2009; 47:1569–1576.
Article30. Ruiz E, Padilla E, Redondo S, Gordillo-Moscoso A, Tejerina T. Kaempferol inhibits apoptosis in vascular smooth muscle induced by a component of oxidized LDL. Eur J Pharmacol. 2006; 529:79–83.
Article31. Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999; 71:479–500.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of L-type Calcium Channel mRNA by the Concentrations of Glucose on the Cell Proliferation in Cultured OLETF Rat Aortic Vascular Smooth Muscle Cells
- Effect of Glucose Concentrations on the Cell Proliferation and Expression of L-type Calcium Channel mRNA in Cultured Rat Aortic Vascular Smooth Muscle Cells
- The effect of anti-hypertensive drugs on DNA synthesis and proliferation of ultured rat aortic smooth muscle cells
- Inhibitory effects of ginseng saponins on c-fos mRNA expression and the proliferation of rat aortic vascular smooth muscle cells stimulated by angiotensin II
- The effect of lovastatin on proliferation of cultured rat mesangial and aortic smooth muscle cells