Korean J Ophthalmol.
2016 Aug;30(4):302-308. 10.3341/kjo.2016.30.4.302.
The Effect of TNF-α Blocker HL036337 and Its Best Concentration to Inhibit Dry Eye Inflammation
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. shadik@yuhs.ac
- 2Noksibcho Hospital, Seoul, Korea.
- 3HanAll Biopharma, Seoul, Korea.
- KMID: 2373991
- DOI: http://doi.org/10.3341/kjo.2016.30.4.302
Abstract
- PURPOSE
Dry eye syndrome is commonly thought of as an inflammatory disease, and we have previously presented data showing the effectiveness of topical TNF-α blocker agents for the treatment of this condition. The purpose of this study was to investigate the effectiveness of the TNF-α blocking agent HL036337 compared to cyclosporine A for the treatment of dry eye induced inflammation in order to establish whether HL036337 represents a more effective method for suppressing inflammation. The efficacy of HL036337 and cyclosporine A was determined using an experimental murine dry eye model.
METHODS
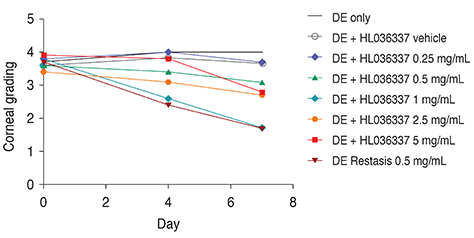
The TNF-α blocker HL036337 is a modified form of TNF receptor I. Using dry eye induced C57BL/6 mice (n = 45), corneal erosion was measured at day 4 and 7 after topical treatment with cyclosporine A or HL036337. To determine the effective treatment dose, 0.25, 0.5, 1, 2.5, and 5 mg/mL of HL036337 were topically administered twice per day to dry eye induced murine corneas for 1 week.
RESULTS
The optimal concentration of the TNF-α blocker HL036337 for treatment of dry eye induced corneal erosion was determined to be 1 mg/mL. Dry eye induced corneal erosion was improved after 1 week with topically applied cyclosporine A and HL036337 at 1 mg/mL.
CONCLUSIONS
HL036337 administered topically at 1 mg/mL effectively improved corneal erosion induced by dry eye. This finding may also suggest that inhibition of TNF-α can improve dry eye syndrome.
MeSH Terms
-
Animals
Cornea/diagnostic imaging
Disease Models, Animal
Dose-Response Relationship, Drug
Dry Eye Syndromes/diagnosis/*drug therapy
Female
Mice
Mice, Inbred C57BL
Microscopy, Acoustic
Ophthalmic Solutions/administration & dosage
Tumor Necrosis Factor-alpha/*antagonists & inhibitors
Ophthalmic Solutions
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008; 115:1982–1988.2. Guo B, Lu P, Chen X, et al. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthalmic Epidemiol. 2010; 17:234–241.3. Kim WJ, Kim HS, Kim MS. Current trends in the recognition and treatment of dry eye: a survey of ophthalmologists. J Korean Ophthalmol Soc. 2007; 48:1614–1622.4. Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003; 110:1412–1419.5. Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006; 4:155–161.6. Song JS, Hyon JY, Lee D, et al. Current practice pattern for dry eye patients in South Korea: a multicenter study. Korean J Ophthalmol. 2014; 28:115–121.7. Brignole F, Pisella PJ, Goldschild M, et al. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000; 41:1356–1363.8. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–589.9. Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009; 28:1023–1027.10. Boehm N, Riechardt AI, Wiegand M, et al. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011; 52:7725–7730.11. Lam H, Bleiden L, de Paiva CS, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009; 147:198–205.e1.12. Chen Y, Zhang X, Yang L, et al. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of d ry eye mice. Mol Med Rep. 2014; 9:2015–2023.13. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003; 3:745–756.14. Cope AP, Londei M, Chu NR, et al. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994; 94:749–760.15. Braun J, Sieper J. Biological therapies in the spondyloarthritides: the current state. Rheumatology (Oxford). 2004; 43:1072–1084.16. Ji YW, Byun YJ, Choi W, et al. Neutralization of ocular surface TNF-α reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci. 2013; 54:7557–7566.17. Barabino S, Shen L, Chen L, et al. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005; 46:2766–2771.18. Moutsopoulos NM, Katsifis GE, Angelov N, et al. Lack of efficacy of etanercept in Sjogren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis. 2008; 67:1437–1443.19. Ramos-Casals M, Tzioufas AG, Stone JH, et al. Treatment of primary Sjogren syndrome: a systematic review. JAMA. 2010; 304:452–460.20. Sankar V, Brennan MT, Kok MR, et al. Etanercept in Sjogren' syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 2004; 50:2240–2245.21. Mah F, Milner M, Yiu S, et al. PERSIST: physician's Evaluation of Restasis(R) Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012; 6:1971–1976.22. Brănişteanu DE, Voicu CM, Cretu A, et al. Adverse reactions of biological therapy for psoriasis. Rev Med Chir Soc Med Nat Iasi. 2015; 119:38–44.23. Ramos-Casals M, Roberto-Perez-Alvarez , Diaz-Lagares C, et al. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010; 9:188–193.24. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000; 47:119–125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent treatment of dry eye
- Inflammatory Factors Predicting Dry Eye Syndrome in a Model Using Osmotic Pressure
- The Concentration of Tear Lysozyme in Normal Subjects and Dry Eye
- The Effects of Dry Eye on the Corneal Thickness Measured by Orbscan and Ultrasonic Pachymetry
- Current Trends in the Recognition and Treatment of Dry Eye: A Survey of Ophthalmologists