Korean J Ophthalmol.
2016 Jun;30(3):172-179. 10.3341/kjo.2016.30.3.172.
Oxidative Stress Levels in Aqueous Humor from High Myopic Patients
- Affiliations
-
- 1Department of Ophthalmology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. schinn@hanmail.net
- 2Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Ophthalmology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2373972
- DOI: http://doi.org/10.3341/kjo.2016.30.3.172
Abstract
- PURPOSE
To compare oxidative stress status in the aqueous humor of highly myopic eyes and control eyes.
METHODS
Aqueous humor samples were collected from 15 highly myopic eyes (high myopia group) and 23 cataractous eyes (control group) during cataract surgery. Central corneal thickness, corneal endothelial cell density, hexagonality of corneal endothelial cells, and cell area of corneal endothelial cells were measured using specular microscopy. Axial length was measured using ultrasound biometry. 8-Hydroxydeoxyguanosine (8-OHdG) and malondialdehyde levels were measured using enzyme-linked immunosorbent assay.
RESULTS
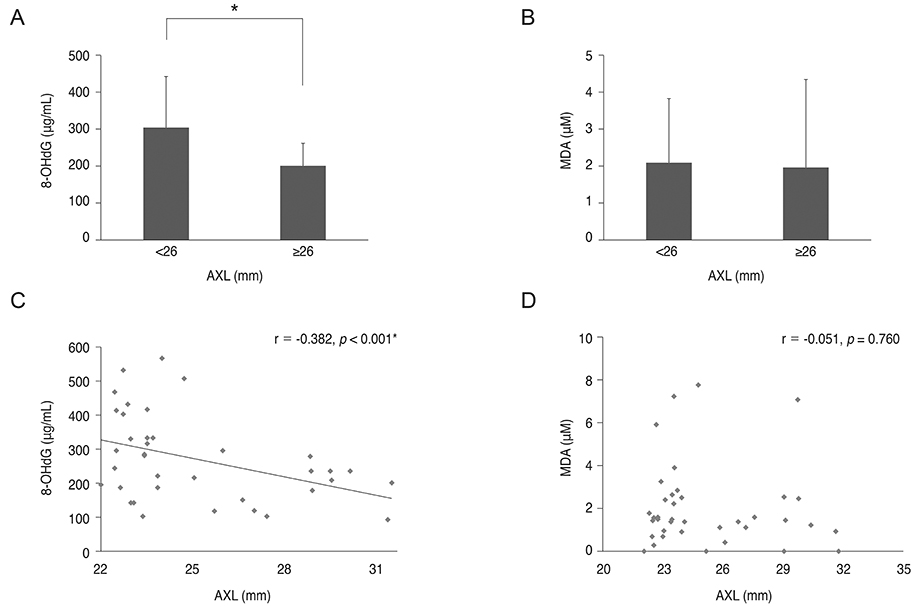
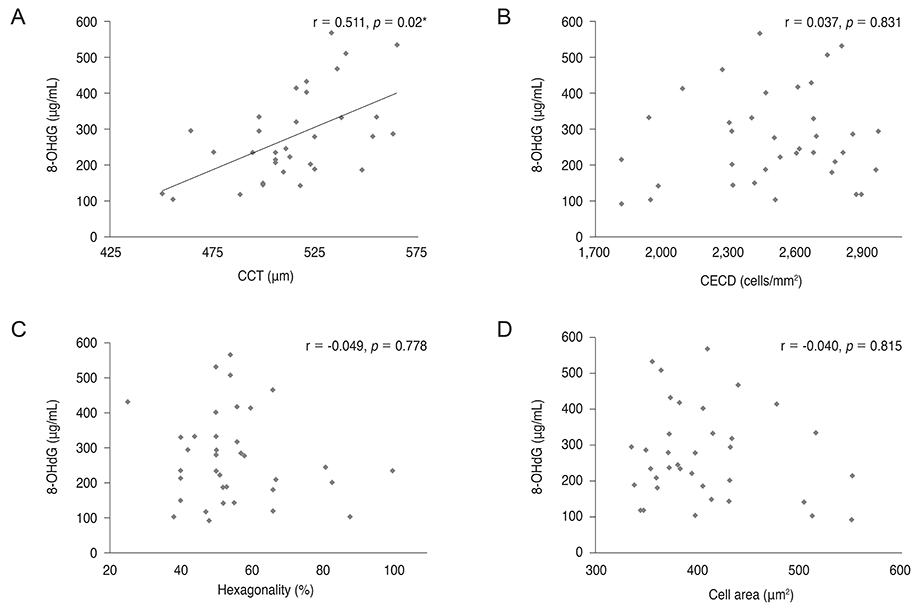
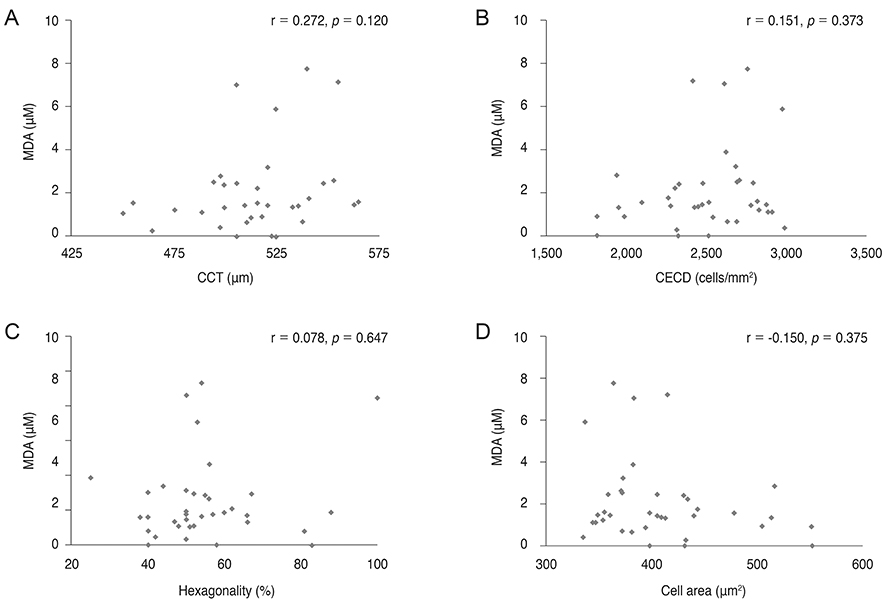
8-OHdG level was lower in the aqueous humor of myopic patients than in that of control group (p = 0.014) and was positively correlated with central corneal thickness and negatively correlated with axial length (r = 0.511, p = 0.02; r = -0.382, p < 0.001). There was no correlation between 8-OHdG level and corneal endothelial cell density, hexagonality, or cell area. Malondialdehyde level did not show any correlation with any parameters evaluated.
CONCLUSIONS
8-OHdG might be a sensitive biomarker for evaluating oxidative stress status in the eye. Oxidative stress level was lower in the aqueous humor of highly myopic eyes compared to that in control eyes, which indicates lower metabolic activity in these eyes.
MeSH Terms
Figure
Reference
-
1. Wakamatsu TH, Dogru M, Matsumoto Y, et al. Evaluation of lipid oxidative stress status in Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 2013; 54:201–210.2. Thiagarajan R, Manikandan R. Antioxidants and cataract. Free Radic Res. 2013; 47:337–345.3. Chen Y, Mehta G, Vasiliou V. Antioxidant defenses in the ocular surface. Ocul Surf. 2009; 7:176–185.4. Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014; 15:243–256.5. Markesbery WR, Lovell MA. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol. 2007; 64:954–956.6. Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004; 339:1–9.7. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004; 142:231–255.8. Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990; 9:515–540.9. Freddo TF. A contemporary concept of the blood-aqueous barrier. Prog Retin Eye Res. 2013; 32:181–195.10. Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009; 88:151–164.11. Silva R. Myopic maculopathy: a review. Ophthalmologica. 2012; 228:197–213.12. Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005; 25:381–391.13. Bosch-Morell F, Sanz A, Diaz-Llopis M, Romero FJ. Lipid peroxidation products in human subretinal fluid. Free Radic Biol Med. 1996; 20:899–903.14. Jia Y, Hu DN, Zhu D, et al. MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels in human aqueous humor: relationship with axial length. Invest Ophthalmol Vis Sci. 2014; 55:3922–3928.15. Shin YJ, Nam WH, Park SE, et al. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol Vis. 2012; 18:2265–2270.16. Jia Y, Hu DN, Zhou J. Human aqueous humor levels of TGF-β2: relationship with axial length. Biomed Res Int. 2014; 2014:258591.17. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III: the Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111:831–836.18. Karbassi M, Khu PM, Singer DM, Chylack LT Jr. Evaluation of lens opacities classification system III applied at the slitlamp. Optom Vis Sci. 1993; 70:923–928.19. Lau LI, Liu CJ, Wei YH. Increase of 8-hydroxy-2'-deoxyguanosine in aqueous humor of patients with exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010; 51:5486–5490.20. Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, et al. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis. 2011; 17:41–46.21. Pedersen L, Hjortdal J, Ehlers N. Central corneal thickness in high myopia. Acta Ophthalmol Scand. 2005; 83:539–542.22. Ortiz S, Mena L, Rio-San Cristobal A, Martin R. Relationships between central and peripheral corneal thickness in different degrees of myopia. J Optom. 2014; 7:44–50.23. Wei W, Fan Z, Wang L, et al. Correlation analysis between central corneal thickness and intraocular pressure in juveniles in Northern China: the Jinan city eye study. PLoS One. 2014; 9:e104842.24. Bothman L. The relation of the basal metabolic rate to progressive axial myopia. Am J Ophthalmol. 1931; 14:918–924.25. Mao JF, Liu SZ, Dou XQ. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int J Ophthalmol. 2012; 5:670–674.26. Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye (Lond). 2002; 16:411–421.27. Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995; 9:1173–1182.28. Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003; 22:657–682.29. Micelli-Ferrari T, Vendemiale G, Grattagliano I, et al. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol. 1996; 80:840–843.30. Wang K, Spector A. Alpha-crystallin can act as a chaperone under conditions of oxidative stress. Invest Ophthalmol Vis Sci. 1995; 36:311–321.31. Zhu XJ, Zhou P, Zhang KK, et al. Epigenetic regulation of αA-crystallin in high myopia-induced dark nuclear cataract. PLoS One. 2013; 8:e81900.32. Cherfan GM, Michels RG, de Bustros S, et al. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol. 1991; 111:434–438.33. Silverstone BZ, Syrkin N, Algur N, Berson D. A metabolic aspect of high myopia. Ann Ophthalmol. 1985; 17:546–551.34. Leveziel N, Yu Y, Reynolds R, et al. Genetic factors for choroidal neovascularization associated with high myopia. Invest Ophthalmol Vis Sci. 2012; 53:5004–5009.35. Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007; 114:216–220.36. Kara A, Akman S, Ozkanlar S, et al. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic Biol Med. 2013; 55:21–26.37. Khalili J, Biloklytska HF. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008; 14:754–760.38. Inomata H, Bill A, Smelser GK. Aqueous humor pathways through the trabecular meshwork and into Schlemm's canal in the cynomolgus monkey (Macaca irus): an electron microscopic study. Am J Ophthalmol. 1972; 73:760–789.39. Schoenwald RD, Ward RL. Relationship between steroid permeability across excised rabbit cornea and octanol-water partition coefficients. J Pharm Sci. 1978; 67:786–788.40. Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm: an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol (Copenh). 1972; 50:295–320.41. Cheng KC, Cahill DS, Kasai H, et al. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and A→C substitutions. J Biol Chem. 1992; 267:166–172.42. Gil HW, Seok SJ, Jeong DS, et al. Plasma level of malondialdehyde in the cases of acute paraquat intoxication. Clin Toxicol (Phila). 2010; 48:149–152.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Analysis of Glucose Concentration in Aqueous Humor in Diabetic Cataract Patients
- Analysis of Aqueous Humor Calcium and Phosphate from Cataract Eyes with and without Diabetes Mellitus
- Studies of the Timolol Effect on Intraocular Pressure and Concentration in Aqueous Humor in the White Rabbit
- Ascorbic Acid Determination in Aqueous and Vitreous Humor of the Rabbit
- The Effect of Aqueous Humor Suppressants on the Duration of Intravitreal Perfluorocarbon Gas Bubble in Rabbits