J Korean Med Sci.
2016 Jun;31(6):879-885. 10.3346/jkms.2016.31.6.879.
Orai1 Expression Is Closely Related with Favorable Prognostic Factors in Clear Cell Renal Cell Carcinoma
- Affiliations
-
- 1Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea. eomm@yonsei.ac.kr
- 2Department of Physiology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Occupational & Environmental Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Department of Global Medical Science, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 6Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 7Department of Pathology, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia.
- KMID: 2373696
- DOI: http://doi.org/10.3346/jkms.2016.31.6.879
Abstract
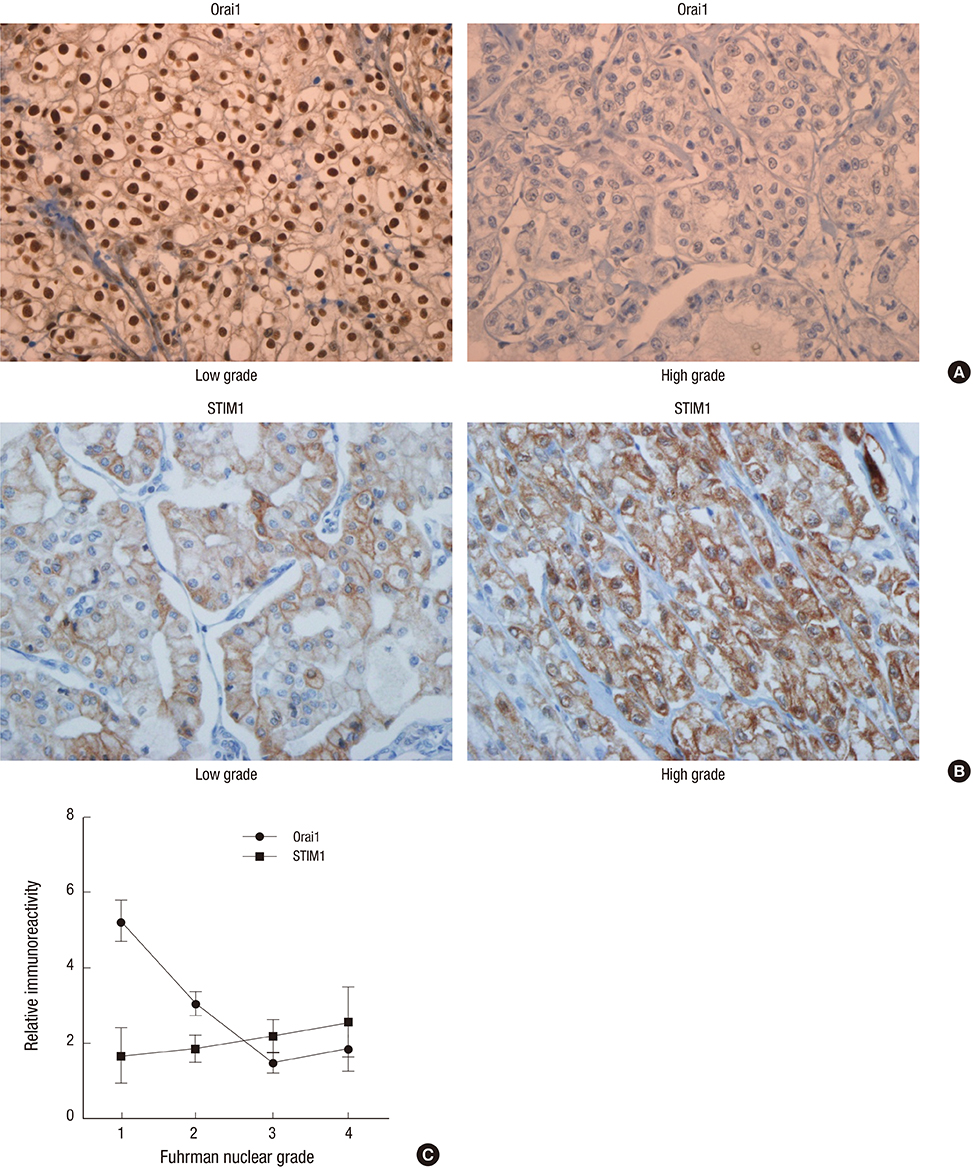
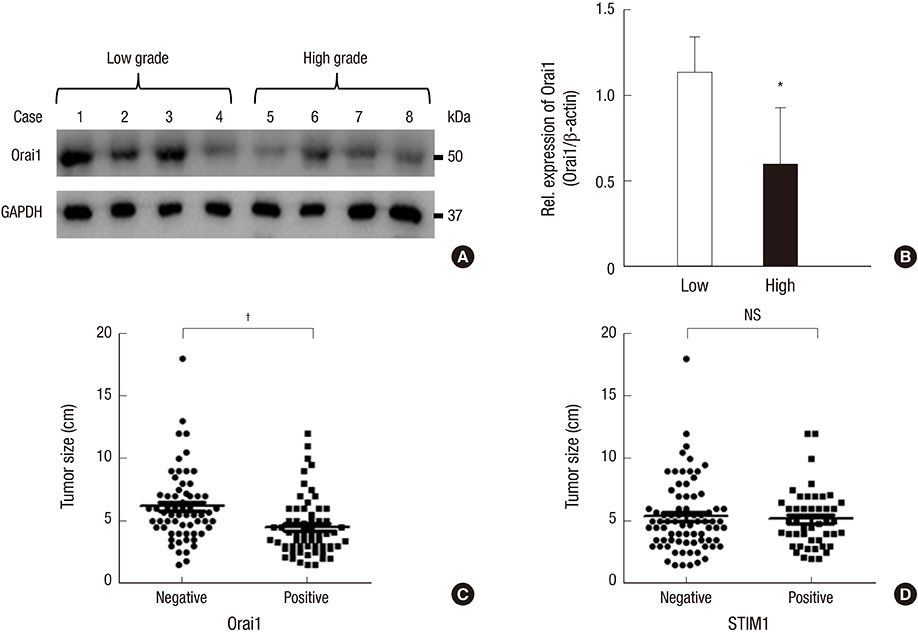
- Store-operated calcium (Ca2+) entry (SOCE) is the principal Ca2+ entry route in non-excitable cells, including cancer cells. We previously demonstrated that Orai1 and STIM1, the molecular components of SOCE, are involved in tumorigenesis of clear cell renal cell carcinoma (CCRCC). However, a clinical relevance of Orai1 and STIM1 expression in CCRCC has been ill-defined. Here, we investigated the expression of Orai1 and STIM1 in CCRCC, and compared their expression with clinico-pathological parameters of CCRCC and the patients' outcome. Immunohistochemical staining for Orai1 and STIM1 was performed on 126 formalin fixed paraffin embedded tissue of CCRCC and western blot analysis for Orai1 was performed on the available fresh tissue. The results were compared with generally well-established clinicopathologic prognostic factors in CCRCC and patient survival. Membrane protein Orai1 is expressed in the nuclei in CCRCC, whereas STIM1 shows the cytosolic expression pattern in immunohistochemical staining. Orai1 expression level is inversely correlated with CCRCC tumor grade, whereas STIM1 expression level is not associated with tumor grade. The higher Orai1 expression is significantly associated with lower Fuhrman nuclear grade, pathologic T stage, and TNM stage and with favorable prognosis. The expression level of STIM1 is not correlated with CCRCC grade and clinical outcomes. Orai1 expression in CCRCC is associated with tumor progression and with favorable prognostic factors. These results suggest that Orai1 is an attractive prognostic marker and therapeutic target for CCRCC.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Blotting, Western
Carcinoma, Renal Cell/*diagnosis/metabolism/*pathology
Female
*Gene Expression Regulation, Neoplastic
Humans
Immunohistochemistry
Kidney Neoplasms/metabolism/*pathology
Male
Middle Aged
Neoplasm Proteins/genetics/metabolism
ORAI1 Protein/genetics/*metabolism
Prognosis
Retrospective Studies
Stromal Interaction Molecule 1/genetics/metabolism
Young Adult
Neoplasm Proteins
ORAI1 Protein
Stromal Interaction Molecule 1
Figure
Reference
-
1. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009; 373:1119–1132.2. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.3. Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005; 23:2763–2771.4. Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012; 53:217–228.5. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529.6. Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev. 2005; 85:757–810.7. Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010; 14:2337–2349.8. Capiod T. The need for calcium channels in cell proliferation. Recent Patents Anticancer Drug Discov. 2013; 8:4–17.9. Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009; 1793:933–940.10. Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986; 7:1–12.11. Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992; 355:353–356.12. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005; 15:1235–1241.13. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006; 443:230–233.14. Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009; 15:124–134.15. Vanden Abeele F, Shuba Y, Roudbaraki M, Lemonnier L, Vanoverberghe K, Mariot P, Skryma R, Prevarskaya N. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation, and role in carcinogenesis. Cell Calcium. 2003; 33:357–373.16. Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol. 2011; 91:753–760.17. Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013; 465:1249–1260.18. Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011; 108:15225–15230.19. Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014; 448:76–82.20. Edge SB. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer;2010. p. 323–328.21. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982; 6:655–663.22. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.23. Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010; 70:6412–6419.24. Zhan ZY, Zhong LX, Feng M, Wang JF, Liu DB, Xiong JP. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int J Clin Exp Pathol. 2015; 8:5080–5088.25. Ritchie MF, Zhou Y, Soboloff J. WT1/EGR1-mediated control of STIM1 expression and function in cancer cells. Front Biosci (Landmark Ed). 2011; 16:2402–2415.26. Ritchie MF, Yue C, Zhou Y, Houghton PJ, Soboloff J. Wilms tumor suppressor 1 (WT1) and early growth response 1 (EGR1) are regulators of STIM1 expression. J Biol Chem. 2010; 285:10591–10596.27. Rivera MN, Haber DA. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer. 2005; 5:699–712.28. Campbell CE, Kuriyan NP, Rackley RR, Caulfield MJ, Tubbs R, Finke J, Williams BR. Constitutive expression of the Wilms tumor suppressor gene (WT1) in renal cell carcinoma. Int J Cancer. 1998; 78:182–188.29. Li X, Wang S, Sitaram RT, Andersson C, Ljungberg B, Li A. Single nucleotide polymorphisms in the Wilms’ tumour gene 1 in clear cell renal cell carcinoma. PLoS One. 2013; 8:e58396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Clinical Stage and Presumptive Prognostic Factors in Renal Cell Carcinoma
- The Expression of Caspase 3 and p21 in Renal Cell Carcinoma
- Differentiation of Chromophobe Renal Cell Carcinoma and Clear Cell Renal Cell Carcinoma by Using Helical CT
- Loss of Nuclear BAP1 Expression Is Associated with High WHO/ISUP Grade in Clear Cell Renal Cell Carcinoma
- Prognostic Significance of E-cadherin Expression in Renal Cell Carcinoma