J Korean Med Sci.
2016 Jun;31(6):866-872. 10.3346/jkms.2016.31.6.866.
Mixed Carcinoma as an Independent Prognostic Factor in Submucosal Invasive Gastric Carcinoma
- Affiliations
-
- 1Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. 20040002@kuh.ac.kr
- 2Department of Surgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2373694
- DOI: http://doi.org/10.3346/jkms.2016.31.6.866
Abstract
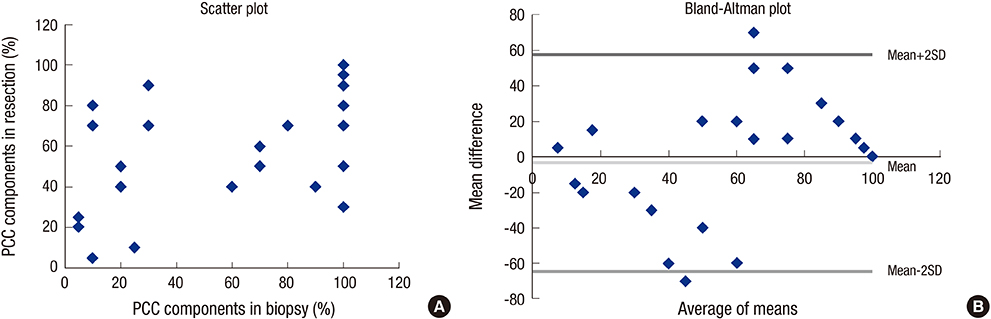
- Mixed carcinoma shows a mixture of glandular and signet ring/poorly cohesive cellular histological components and the prognostic significance of each component is not fully understood. This study aimed to investigate the significance of the poorly cohesive cellular histological component as a risk factor for lymph node metastasis and to examine the diagnostic reliability of endoscopic biopsy. Clinicopathologic characteristics of 202 patients who underwent submucosal invasive gastric carcinoma resection with lymph node dissection in 2005-2012 were reviewed. Mixed carcinoma accounted for 27.2% (56/202) of cases. The overall prevalence of lymph node metastasis was 17.3% (35/202). Lymphatic invasion (P < 0.001), family history of carcinoma (P = 0.025), tumor size (P = 0.004), Lauren classification (P = 0.042), and presence of any poorly cohesive cellular histological component (P = 0.021) positively correlated with the lymph node metastasis rate on univariate analysis. Multivariate analyses revealed lymphatic invasion, family history of any carcinoma, and the presence of any poorly cohesive cellular histological component to be significant and independent factors related to lymph node metastasis. Review of preoperative biopsy slides showed that preoperative biopsy demonstrated a sensitivity of 63.6% and a specificity of 100% in detecting the presence of the poorly cohesive cellular histological component, compared with gastrectomy specimens. The presence of any poorly cohesive cellular histological component was an independent risk factor associated with lymph node metastasis in submucosal invasive gastric carcinoma. Endoscopic biopsy had limited value in predicting the presence and proportion of the poorly cohesive cellular histologic component due to the heterogeneity of mixed carcinoma.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Current Status of Endoscopic Resection of Early Gastric Cancer in Korea
Hwoon-Yong Jung
Korean J Gastroenterol. 2017;70(3):121-127. doi: 10.4166/kjg.2017.70.3.121.A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi, Hee Kyung Chang, Soomin Ahn, Mee Soo Chang, Song-Hee Han, Yoonjin Kwak, An Na Seo, Sung Hak Lee, Mee-Yon Cho
J Pathol Transl Med. 2023;57(1):1-27. doi: 10.4132/jptm.2022.12.23.
Reference
-
1. Yi HW, Kim SM, Kim SH, Shim JH, Choi MG, Lee JH, Noh JH, Sohn TS, Bae JM, Kim S. Complications leading reoperation after gastrectomy in patients with gastric cancer: frequency, type, and potential causes. J Gastric Cancer. 2013; 13:242–246.2. Jeon YW, Han SI, Jeon CE, Kim JJ, Park SM. Quality of life in patients with stomach cancer after operation. J Korean Gastric Cancer Assoc. 2004; 4:27–31.3. ASGE Technology Committee. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008; 68:11–18.4. Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006; 22:561–569.5. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014; 14:87–104.6. Hiki Y, Shimao H, Mieno H, Sakakibara Y, Kobayashi N, Saigenji K. Modified treatment of early gastric cancer: evaluation of endoscopic treatment of early gastric cancers with respect to treatment indication groups. World J Surg. 1995; 19:517–522.7. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.8. Lauwers GY, Carneiro F, Graham DY, Curado MP, Franceschi S, Montgomery E. Tumours of the stomach. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: IARC Press;2010. p. 48–58.9. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.10. Fenoglio-Preiser C, Carneiro F, Correa P, Guilford P, Lambert R, Megraud F, Munoz N, Powell SM, Rugge M, Sasako M, et al. Tumours of the stomach. In : Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System: WHO Classification of Tumours. 3rd ed. Lyon: IARC Press;2000. p. 37–68.11. Gotoda T, Sasako M, Ono H, Katai H, Sano T, Shimoda T. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg. 2001; 88:444–449.12. Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009; 12:148–152.13. Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009; 41:498–503.14. Kurihara N, Kubota T, Otani Y, Ohgami M, Kumai K, Sugiura H, Kitajima M. Lymph node metastasis of early gastric cancer with submucosal invasion. Br J Surg. 1998; 85:835–839.15. Kupelian PA, Kupelian VA, Witte JS, Macklis R, Klein EA. Family history of prostate cancer in patients with localized prostate cancer: an independent predictor of treatment outcome. J Clin Oncol. 1997; 15:1478–1480.16. Hata K, Shinozaki M, Toyoshima O, Toyoshima A, Matsumoto S, Saisho T, Tsurita G. Impact of family history of gastric cancer on colorectal neoplasias in young Japanese. Colorectal Dis. 2013; 15:42–46.17. Minami Y, Kawai M, Fujiya T, Suzuki M, Noguchi T, Yamanami H, Kakugawa Y, Nishino Y. Family history, body mass index and survival in Japanese patients with stomach cancer: a prospective study. Int J Cancer. 2015; 136:411–424.18. Wong VC, Ko JM, Qi RZ, Li PJ, Wang LD, Li JL, Chan YP, Chan KW, Stanbridge EJ, Lung ML. Abrogated expression of DEC1 during oesophageal squamous cell carcinoma progression is age- and family history-related and significantly associated with lymph node metastasis. Br J Cancer. 2011; 104:841–849.19. Yu J, Fu B, Zhao Q. Family history of malignant neoplasm and its relation with clinicopathologic features of gastric cancer patients. World J Surg Oncol. 2013; 11:201.20. Shim CN, Chung H, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Early gastric cancer with mixed histology predominantly of differentiated type is a distinct subtype with different therapeutic outcomes of endoscopic resection. Surg Endosc. 2015; 29:1787–1794.21. Shimizu H, Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M, et al. The decision criterion of histological mixed type in “T1/T2” gastric carcinoma--comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012; 105:800–804.22. Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009; 41:427–432.23. Lee JH, Choi IJ, Han HS, Kim YW, Ryu KW, Yoon HM, Eom BW, Kim CG, Lee JY, Cho SJ, et al. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol. 2015; 22:1813–1819.24. Kozuki T, Yao T, Nakamura S, Matsumoto T, Tsuneyoshi M. Differences in p53 and cadherin-catenin complex expression between histological subtypes in diffusely infiltrating gastric carcinoma. Histopathology. 2002; 41:56–64.25. Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015; 18:618–626.26. Joo M, Kim KM. Histologic discrepancy between gastric biopsy and resection specimen in the era of endoscopic treatment for early gastric cancer. Korean J Gastroenterol. 2014; 64:256–259.27. Shim CN, Kim H, Kim DW, Chung HS, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Clinicopathologic factors and outcomes of histologic discrepancy between differentiated and undifferentiated types after endoscopic resection of early gastric cancer. Surg Endosc. 2014; 28:2097–2105.28. Carneiro F, Machado JC, Nabais S, Santos CM, Sobrinho Simões M. Mixed carcinoma of the stomach: a clinicopathological entity. Histopathology. 2003; 43:94–95.29. Honda T, Tamura G, Endoh Y, Nishizuka S, Kawata S, Motoyama T. Expression of tumor suppressor and tumor-related proteins in differentiated carcinoma, undifferentiated carcinoma with tubular component and pure undifferentiated carcinoma of the stomach. Jpn J Clin Oncol. 2005; 35:580–586.30. Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008; 452:525–534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of submucosal gastric lymphoepithelioma-like carcinoma
- Predictors of Lymph Node Metastasis in Submucosal Gastric Carcinomas
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- Predictors of Lymph Node Metastasis in Submucosal Gastric Carcinomas
- Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor