J Korean Med Sci.
2016 May;31(5):674-681. 10.3346/jkms.2016.31.5.674.
Knowledge and Perception about Clinical Research Shapes Behavior: Face to Face Survey in Korean General Public
- Affiliations
-
- 1Asan Medical Center, Clinical Trial Center, Seoul, Korea. twkimmd@amc.seoul.kr
- 2Asan Medical Center, Human Research Protection Center, Seoul, Korea.
- 3Department of Psychiatry, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Asan Medical Center, Clinical Research Center, Seoul, Korea.
- 5Duke Global Proof-of-Concept (POC) Research Network, Duke Clinical Research Unit (DCRU) & Duke Clinical Research Institute (DCRI), Duke University, Durham, NC, USA.
- 6Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC, USA.
- 7Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2373662
- DOI: http://doi.org/10.3346/jkms.2016.31.5.674
Abstract
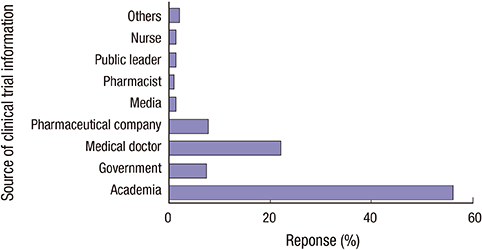
- Considering general public as potential patients, identifying factors that hinder public participation poses great importance, especially in a research environment where demands for clinical trial participants outpace the supply. Hence, the aim of this study was to evaluate knowledge and perception about clinical research in general public. A total of 400 Seoul residents with no previous experience of clinical trial participation were selected, as representative of population in Seoul in terms of age and sex. To minimize selection bias, every fifth passer-by was invited to interview, and if in a cluster, person on the very right side was asked. To ensure the uniform use of survey, written instructions have been added to the questionnaire. Followed by pilot test in 40 subjects, the survey was administered face-to-face in December 2014. To investigate how perception shapes behavior, we compared perception scores in those who expressed willingness to participate and those who did not. Remarkably higher percentage of responders stated that they have heard of clinical research, and knew someone who participated (both, P < 0.001) compared to India. Yet, the percentage of responders expressed willingness to participate was 39.3%, a significantly lower rate than the result of the India (58.9% vs. 39.3%, P < 0.001). Treatment benefit was the single most influential reason for participation, followed by financial gain. Concern about safety was the main reason for refusal, succeeded by fear and lack of trust. Public awareness and educational programs addressing these negative perceptions and lack of knowledge will be effective in enhancing public engaged in clinical research.
Keyword
MeSH Terms
Figure
Reference
-
1. Hussain-Gambles M, Atkin K, Leese B. South Asian participation in clinical trials: the views of lay people and health professionals. Health Policy. 2006; 77:149–165.2. Rogers WA. Evidence based medicine and justice: a framework for looking at the impact of EBM upon vulnerable or disadvantaged groups. J Med Ethics. 2004; 30:141–145.3. Lang T, Siribaddana S. Clinical trials have gone global: is this a good thing? PLoS Med. 2012; 9:e1001228.4. George M, Selvarajan S, S SK, Dkhar SA, Chandrasekaran A. Globalization of clinical trials - where are we heading? Curr Clin Pharmacol. 2013; 8:115–123.5. Casas JP, Cubillos-Garzón LA, Morillo CA. Regional pathologies and globalization of clinical trials: has the time for regional trials arrived? Circulation. 2003; 107:e194.6. Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009; 360:816–823.7. Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008; 7:13–14.8. Drain PK, Robine M, Holmes KK, Bassett IV. Trial watch: global migration of clinical trials. Nat Rev Drug Discov. 2014; 13:166–167.9. ClinicalTrials.gov (US). See studies on map. accessed on 5 January 2015. Available at https://clinicaltrials.gov/ct2/search/map.10. Korea National Enterprise for Clinical Trials. accessed on 20 October 2014. Available at http://www.konect.or.kr/.11. Woolley M, Propst SM. Public attitudes and perceptions about health-related research. JAMA. 2005; 294:1380–1384.12. Burt T, Dhillon S, Sharma P, Khan D, Mv D, Alam S, Jain S, Alapati B, Mittal S, Singh P. PARTAKE survey of public knowledge and perceptions of clinical research in India. PLoS One. 2013; 8:e68666.13. van der Heyden MA, van de Ven T, Opthof T. Fraud and misconduct in science: the stem cell seduction: implications for the peer-review process. Neth Heart J. 2009; 17:25–29.14. Hvistendahl M. Drug development. Corruption and research fraud send big chill through big pharma in China. Science. 2013; 341:445–446.15. Kim JW, Kim SJ, Chung YH, Kwon JH, Lee HJ, Chung YJ, Kim YJ, Oh DY, Lee SH, Kim DW, et al. Cancer patients’ awareness of clinical trials, perceptions on the benefit and willingness to participate: Korean perspectives. Br J Cancer. 2008; 99:1593–1599.16. Lee SJ, Park LC, Lee J, Kim S, Choi MK, Hong JY, Park S, Maeng CH, Chang W, Kim YS, et al. Unique perception of clinical trials by Korean cancer patients. BMC Cancer. 2012; 12:594.17. Michaels M, Weiss ES, Guidry JA, Blakeney N, Swords L, Gibbs B, Yeun S, Rytkonen B, Goodman R, Jarama SL, et al. The promise of community-based advocacy and education efforts for increasing cancer clinical trials accrual. J Cancer Educ. 2012; 27:67–74.18. Mackenzie IS, Wei L, Rutherford D, Findlay EA, Saywood W, Campbell MK, Macdonald TM. Promoting public awareness of randomised clinical trials using the media: the ‘Get Randomised’ campaign. Br J Clin Pharmacol. 2010; 69:128–135.19. Seoul Metropolitan Government. Seoul statistics by category: population. accessed on 20 October 2014. Available at http://english.seoul.go.kr/get-to-know-us/statistics-of-seoul/seoul-statistics-by-category/.20. Korea Centers for Disease Control and Prevention. Attitudes and Perceptions of Clinical Trials in Public: Results from a Population-Based Survey [Unpublished Report]. Cheongwon: Korea Centers for Disease Control and Prevention;2009. p. 1–4.21. Abbott D, Califf R, Morrison BW, Pierre C, Bolte J, Chakraborty S. Cycle time metrics for multisite clinical trials in the United States. Ther Innov Regul Sci. 2013; 47:152–160.22. The Center for Information and Study on Clinical Research Participation (US). 2013 International Survey on Public and Patient Attitudes about, and Experiences with, Clinical Research Studies [Unpublished Report]. Boston, MA: The Center for Information and Study on Clinical Research Participation;2013.23. Happer C, Philo G. The role of the media in the construction of public belief and social change. J Soc Polit Psych. 2013; 1:321–336.24. Kim H. Exercise rehabilitation for smartphone addiction. J Exerc Rehabil. 2013; 9:500–505.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Face Mask Usage, Knowledge and Behavior of Face Mask Usage in Older Adults Living Alone in the COVID-19 Era
- Neurobiology of Facial Perception and Facial Affect Recognition
- Types of perception toward non-face-to-face clinical practice among nursing students
- Non-face-to-face Treatment in Korea: Suggestions for Essential Conditions
- A Case of Prosopometamorphopsia Restricted to the Nose and Mouth with Right Medial Temporooccipital Lobe Infarction that Included the Fusiform Face Area