Ann Lab Med.
2016 May;36(3):259-262. 10.3343/alm.2016.36.3.259.
The First Korean Family With Hereditary Gelsolin Amyloidosis Caused by p.D214Y Mutation in the GSN Gene
- Affiliations
-
- 1Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Seoul, Korea. kimjw@skku.edu
- 2Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- 3Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bjkim@skku.edu
- 4Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2373537
- DOI: http://doi.org/10.3343/alm.2016.36.3.259
Abstract
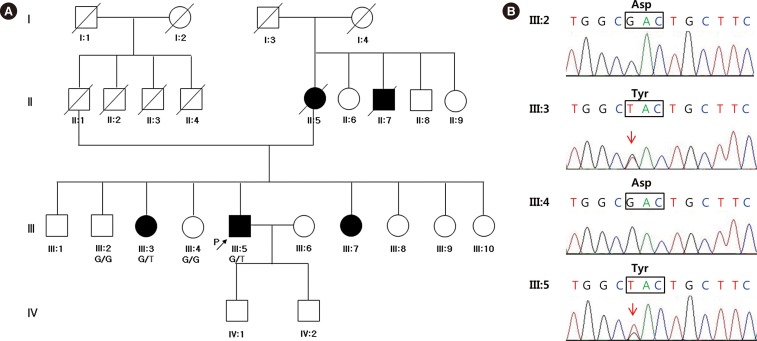
- Hereditary gelsolin amyloidosis (HGA) is an autosomal dominant hereditary disease characterized by corneal lattice dystrophy, peripheral neuropathy, and cutis laxa. So far, no Korean patients with HGA have been reported. A 58-yr-old man presented with involuntary facial twitching, progressive bilateral facial weakness, and tongue atrophy. His mother, maternal uncle, two sisters, and son suffered from the same symptoms. Electrophysiological studies revealed signs of chronic denervation in the cervical and lumbar regions, mild sympathetic autonomic dysfunction, and bilateral facial nerve dysfunction. Diagnostic whole-exome sequencing (WES) revealed a p.D214Y heterozygous mutation in the gelsolin gene in affected members. We present the first report of a Korean family with HGA diagnosed by WES. WES facilitated a clinical diagnosis of HGA in patients with undiagnosed neuropathies.
MeSH Terms
Figure
Reference
-
1. Meretoja J. Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms. A previously unrecognized heritable syndrome. Ann Clin Res. 1969; 1:314–324. PMID: 4313418.2. Meretoja J. Genetic aspects of familial amyloidosis with corneal lattice dystrophy and cranial neuropathy. Clin Genet. 1973; 4:173–185. PMID: 4543600.
Article3. Maury CP, Kere J, Tolvanen R, de la Chapelle A. Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene. FEBS Lett. 1990; 276:75–77. PMID: 2176164.
Article4. Taira M, Ishiura H, Mitsui J, Takahashi Y, Hayashi T, Shimizu J, et al. Clinical features and haplotype analysis of newly identified Japanese patients with gelsolin-related familial amyloidosis of Finnish type. Neurogenetics. 2012; 13:237–243. PMID: 22622774.
Article5. Maury CP, Liljeström M, Boysen G, Törnroth T, de la Chapelle A, Nurmiaho-Lassila EL. Danish type gelsolin related amyloidosis: 654G-T mutation is associated with a disease pathogenetically and clinically similar to that caused by the 654G-A mutation (familial amyloidosis of the Finnish type). J Clin Pathol. 2000; 53:95–99. PMID: 10767822.
Article6. de la Chapelle A, Tolvanen R, Boysen G, Santavy J, Bleeker-Wagemakers L, Maury CP, et al. Gelsolin-derived familial amyloidosis caused by asparagine or tyrosine substitution for aspartic acid at residue 187. Nat Genet. 1992; 2:157–160. PMID: 1338910.
Article7. Solomon JP, Page LJ, Balch WE, Kelly JW. Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit Rev Biochem Mol Biol. 2012; 47:282–296. PMID: 22360545.
Article8. Ikeda M, Mizushima K, Fujita Y, Watanabe M, Sasaki A, Makioka K, et al. Familial amyloid polyneuropathy (Finnish type) in a Japanese family: Clinical features and immunocytochemical studies. J Neurol Sci. 2007; 252:4–8. PMID: 17097682.
Article9. Lüttmann RJ, Teismann I, Husstedt IW, Ringelstein EB, Kuhlenbäumer G. Hereditary amyloidosis of the Finnish type in a German family: clinical and electrophysiological presentation. Muscle Nerve. 2010; 41:679–684. PMID: 20229579.
Article10. Ardalan MR, Shoja MM, Kiuru-Enari S. Amyloidosis-related nephrotic syndrome due to a G654A gelsolin mutation: the first report from the Middle East. Nephrol Dial Transplant. 2007; 22:272–275. PMID: 16998221.
Article11. de la Chapelle A, Kere J, Sack GH Jr, Tolvanen R, Maury CP. Familial amyloidosis, Finnish type: G654----a mutation of the gelsolin gene in Finnish families and an unrelated American family. Genomics. 1992; 13:898–901. PMID: 1322359.12. Chastan N, Baert-Desurmont S, Saugier-Veber P, Dérumeaux G, Cabot A, Frébourg T, et al. Cardiac conduction alterations in a French family with amyloidosis of the Finnish type with the p.Asp187Tyr mutation in the GSN gene. Muscle Nerve. 2006; 33:113–119. PMID: 16258946.13. Paunio T, Sunada Y, Kiuru S, Makishita H, Ikeda S, Weissenbach J, et al. Haplotype analysis in gelsolin-related amyloidosis reveals independent origin of identical mutation (G654A) of gelsolin in Finland and Japan. Hum Mutat. 1995; 6:60–65. PMID: 7550233.14. Solari HP, Ventura MP, Antecka E, Belfort Junior R, Burnier MN Jr. Danish type gelsolin-related amyloidosis in a Brazilian family: case reports. Arq Bras Oftalmol. 2011; 74:286–288. PMID: 22068858.
Article15. Sethi S, Theis JD, Quint P, Maierhofer W, Kurtin PJ, Dogan A, et al. Renal amyloidosis associated with a novel sequence variant of gelsolin. Am J Kidney Dis. 2013; 61:161–166. PMID: 22938848.
Article16. Efebera YA, Sturm A, Baack EC, Hofmeister CC, Satoskar A, Nadasdy T, et al. Novel gelsolin variant as the cause of nephrotic syndrome and renal amyloidosis in a large kindred. Amyloid. 2014; 21:110–112. PMID: 24601799.
Article17. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010; 38:e164. PMID: 20601685.
Article18. Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013; 34:E2393–E2402. PMID: 23843252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Korean family with AGel amyloidosis presenting with progressive facial and bulbar palsies
- Lattice Corneal Dystrophy, Gelsolin Type: The First Case Report in Korea
- Clinical Features and Brain MRI Findings in Korean Patients with AGel Amyloidosis
- A case of hereditary pancreatitis with a N29I mutation in the cationic trypsinogen gene
- Three Cases of Hereditary Pancreatitis in Two Households in the Same Family Associated with R122H Mutation in Cationic Trypsinogen Gene