Korean J Ophthalmol.
2017 Apr;31(2):108-114. 10.3341/kjo.2017.31.2.108.
Dexamethasone Intravitreal Implant Rescue Treatment for Bevacizumab Refractory Macular Edema Secondary to Branch Retinal Vein Occlusion
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. hjkoh@yuhs.ac
- KMID: 2373466
- DOI: http://doi.org/10.3341/kjo.2017.31.2.108
Abstract
- PURPOSE
To evaluate the prognostic factors and outcomes of dexamethasone intravitreal implant (DEX implant) for intravitreal bevacizumab refractory macular edema secondary to branch retinal vein occlusion (BRVO).
METHODS
This was a retrospective, interventional case series. Medical records were reviewed, and a total of 38 eyes that were treated with DEX implant for macular edema secondary to BRVO that did not respond to at least two consecutive intravitreal bevacizumab injections (IBIs) were included. Best-corrected visual acuity (BCVA), central subfield macular thickness, and central subfoveal choroidal thickness were evaluated at baseline, 2 months, and 6 months after DEX implantation.
RESULTS
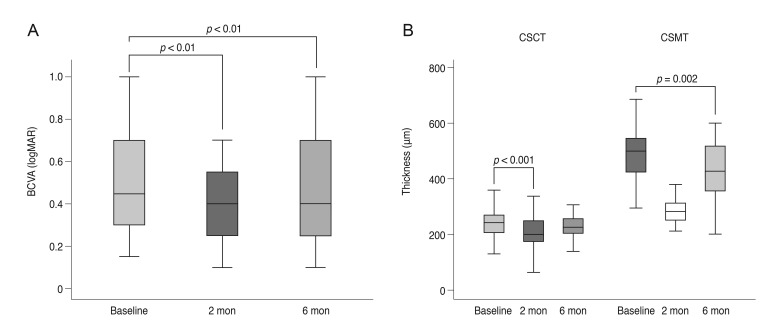
Patients had undergone an average of 6.32 ± 4.66 prior IBI treatments. The average BCVA improved from 0.53 ± 0.26 to 0.41 ± 0.25 and 0.44 ± 0.23 logarithm of the minimal angle of resolution (logMAR) at 2 and 6 months, respectively (p < 0.001). The average central subfield macular thickness was 504.00 ± 121.54 µm at baseline and changed to 293.21 ± 74.17 µm and 427.28 ± 119.57 µm at 2 and 6 months, respectively (p < 0.001 and p = 0.002). Average central subfoveal choroidal thickness was 237.46 ± 92.21 µm at baseline and changed to 204.75 ± 74.74 µm and 226.86 ± 90.77 µm at 2 and 6 months, respectively (p < 0.001 and p = 0.455). Twenty-two eyes (58%) gained ≥0.1 logMAR at 2 months, while 16 eyes showed no improvement. Low BCVA at symptom presentation, low baseline BCVA, and shorter duration of macular edema were correlated with increased BCVA after treatment.
CONCLUSIONS
The DEX implant improves functional and anatomical outcomes for up to 6 months in about half of the patients treated with IBI refractory macular edema secondary to BRVO, particularly in patients with low initial and baseline BCVA.
MeSH Terms
Figure
Reference
-
1. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114:1243–1247. PMID: 8859084.2. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–799. PMID: 18362932.
Article3. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–1101.e5. PMID: 20430447.
Article4. Shilling JS, Jones CA. Retinal branch vein occlusion: a study of argon laser photocoagulation in the treatment of macular oedema. Br J Ophthalmol. 1984; 68:196–198. PMID: 6365157.
Article5. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009; 127:1115–1128. PMID: 19752420.6. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009; 127:1101–1114. PMID: 19752419.7. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102–1112.e1. PMID: 20398941.8. Varma R, Bressler NM, Suner I, et al. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology. 2012; 119:2108–2118. PMID: 22817833.9. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–1146.e3. PMID: 20417567.
Article10. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011; 118:1594–1602. PMID: 21684606.
Article11. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011; 118:2041–2049. PMID: 21715011.
Article12. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014; 121:209–219. PMID: 24112944.13. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016; 123:330–336. PMID: 26522708.14. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–456. PMID: 19074916.15. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012; 119:1388–1398. PMID: 22555112.16. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011; 118:2453–2460. PMID: 21764136.17. Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991; 98(5 Suppl):741–756. PMID: 2062510.18. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–845. PMID: 21211846.
Article19. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–815. PMID: 19232559.
Article20. Joshi L, Yaganti S, Gemenetzi M, et al. Dexamethasone implants in retinal vein occlusion: 12-month clinical effectiveness using repeat injections as-needed. Br J Ophthalmol. 2013; 97:1040–1044. PMID: 23686324.
Article21. Capone A Jr, Singer MA, Dodwell DG, et al. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014; 34:342–351. PMID: 23846381.
Article22. Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008; 22:42–48. PMID: 16826241.
Article23. Noma H, Funatsu H, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009; 116:87–93. PMID: 19118700.
Article24. Fonollosa A, Garcia-Arumi J, Santos E, et al. Vitreous levels of interleukine-8 and monocyte chemoattractant protein-1 in macular oedema with branch retinal vein occlusion. Eye (Lond). 2010; 24:1284–1290. PMID: 20111061.
Article25. Sohn HJ, Han DH, Lee DY, Nam DH. Changes in aqueous cytokines after intravitreal triamcinolone versus bevacizumab for macular oedema in branch retinal vein occlusion. Acta Ophthalmol. 2014; 92:e217–e224. PMID: 23889803.
Article26. Park SP, Ahn JK. Changes of aqueous vascular endothelial growth factor and interleukin-6 after intravitreal triamcinolone for branch retinal vein occlusion. Clin Exp Ophthalmol. 2008; 36:831–835. PMID: 19278477.
Article27. Lee EK, Han JM, Hyon JY, Yu HG. Changes in choroidal thickness after intravitreal dexamethasone implant injection in retinal vein occlusion. Br J Ophthalmol. 2015; 99:1543–1549. PMID: 25883084.
Article28. Alshahrani ST, Dolz-Marco R, Gallego-Pinazo R, et al. Intravitreal dexamethasone implant for the treatment of refractory macular edema in retinal vascular diseases: results of the KKESH International Collaborative Retina Study Group. Retina. 2016; 36:131–136. PMID: 26079477.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
- Intravitreal Dexamethasone Implant for Macular Edema in Branch Retinal Vein Occlusion According to Previous Responses to Bevacizumab
- A Comparison of Three Intravitreal Modalities of Branch Retinal Vein Occlusion Macular Edema
- Predictors of Dexamethasone Response of Residual Edema by Branch Retinal Vein Occlusion after Bevacizumab Injection
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema