Korean J Gastroenterol.
2015 Aug;66(2):75-84. 10.4166/kjg.2015.66.2.75.
RUNX3 Methylation, Loss of RUNX3 Expression and Clinicopathologic Findings according to Helicobacter pylori CagA in Gastric Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. shimkn@ewha.ac.kr
- 2Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Center for Health Promotion, Samsung Medical Center, Seoul, Korea.
- KMID: 2373318
- DOI: http://doi.org/10.4166/kjg.2015.66.2.75
Abstract
- BACKGROUND/AIMS
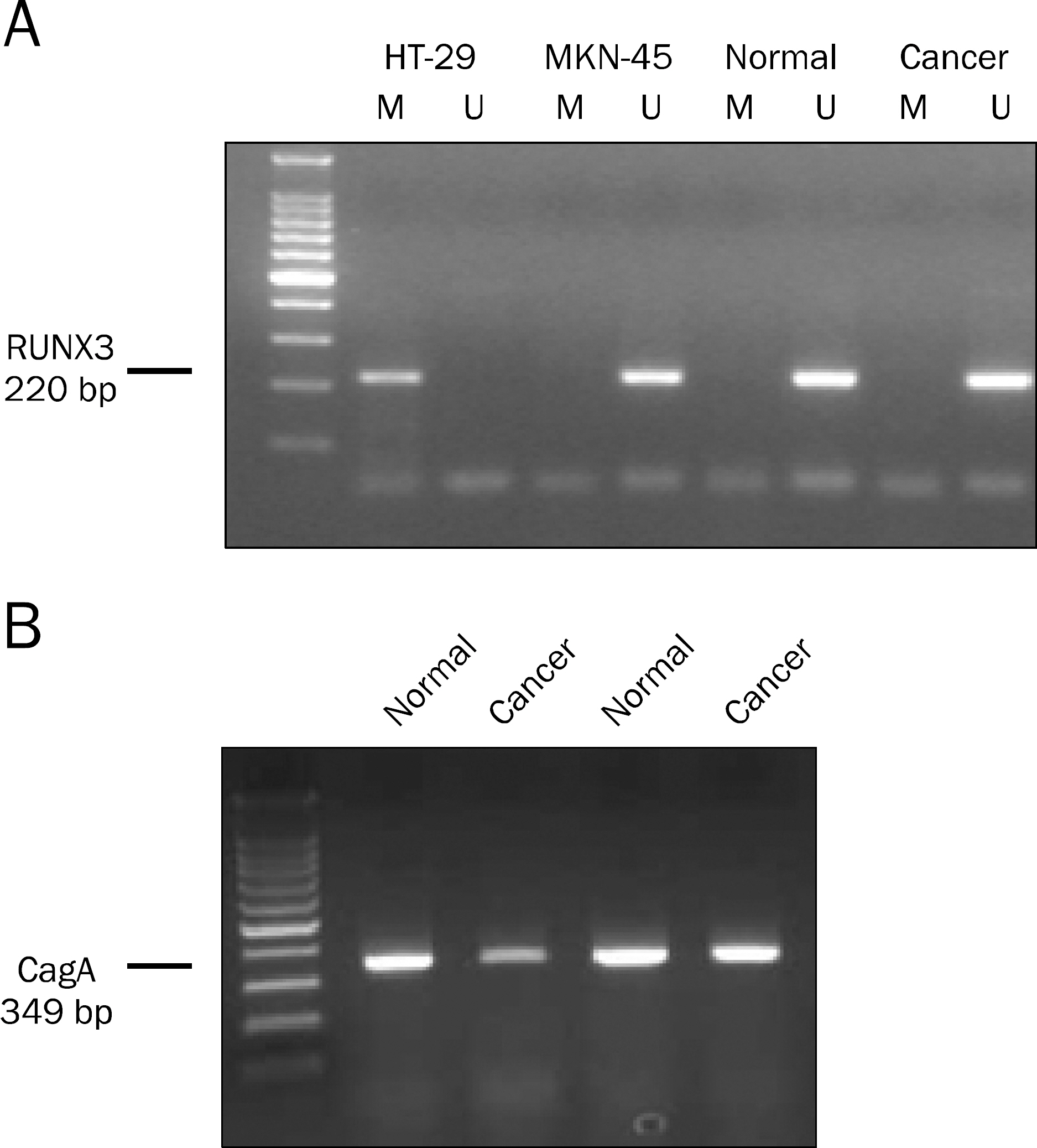
Helicobacter pylori cytotoxin-associated gene A (CagA) has been suggested to be involved in the inactivation of Runt-related transcription factor 3 (RUNX3), a known gastric carcinoma tumor suppressor gene. It remains unclear how H. pylori CagA initiates or maintains RUNX3 promoter methylation and inactivates its protein expression in gastric carcinoma.
METHODS
RUNX3 promoter methylation status, RUNX3 expression, and H. pylori CagA were investigated in 76 sample pairs of gastric carcinoma tissue. The patients' medical records were reviewed. The association between RUNX3 methylation or loss of RUNX3 expression and clinicopathologic variables according to H. pylori CagA status were investigated.
RESULTS
In gastric carcinoma patients with H. pylori CagA-positive infection, RUNX3 methylation did not show association with lymphatic invasion, venous invasion, and TNM stages. However RUNX3 methylation was observed more frequently in poorly differentiated adenocarcinoma and signet ring cell carcinoma (77.8% vs. 20.0%, p=0.023) in early stage. In gastric carcinoma patients with H. pylori CagA-positive infection, loss of RUNX3 expression did not show association with lymphatic invasion, venous invasion, and TNM stages. However loss of RUNX3 expression was observed more frequently in early gastric carcinoma than in advanced gastric carcinoma (84.2% vs. 75.0%, p=0.51), but this difference was not significant.
CONCLUSIONS
In gastric carcinoma patients with H. pylori CagA-positive infection, RUNX3 methylation or loss of RUNX3 expression did not show correlation with lymphovascular invasion and TNM stages. In early gastric carcinoma patients with H. pylori CagA-positive infection, RUNX3 methylation was observed more in poorly differentiated adenocarcinoma and signet ring cell carcinoma.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antigens, Bacterial/*metabolism
Bacterial Proteins/*metabolism
Cell Line, Tumor
Core Binding Factor Alpha 3 Subunit/genetics/*metabolism
Female
*Gene Expression Regulation, Neoplastic
Helicobacter Infections/complications/microbiology
Helicobacter pylori/isolation & purification/*metabolism
Humans
Immunohistochemistry
Lymphatic Metastasis
Male
Methylation
Middle Aged
Neoplasm Staging
Promoter Regions, Genetic
Retrospective Studies
Stomach Neoplasms/complications/microbiology/*pathology
Antigens, Bacterial
Bacterial Proteins
Core Binding Factor Alpha 3 Subunit
Figure
Reference
-
References
1. Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005; 128:1567–1578.2. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994; 61:1–241.3. Wang C, Yuan Y, Hunt RH. The association between Helicobacter pylori infection and early gastric cancer: a metaanalysis. Am J Gastroenterol. 2007; 102:1789–1798.4. Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993; 90:5791–5795.5. Asahi M, Azuma T, Ito S, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000; 191:593–602.6. Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008; 10:1573–1581.7. Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003; 125:1636–1644.
Article8. Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002; 109:113–124.
Article9. Ito K, Liu Q, Salto-Tellez M, et al. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer Res. 2005; 65:7743–7750.
Article10. Fan XY, Hu XL, Han TM, et al. Association between RUNX3 promoter methylation and gastric cancer: a metaanalysis. BMC Gastroenterol. 2011; 11:92.
Article11. Kitajima Y, Ohtaka K, Mitsuno M, et al. Helicobacter pylori infection is an independent risk factor for Runx3 methylation in gastric cancer. Oncol Rep. 2008; 19:197–202.12. Ito K, Chuang LS, Ito T, et al. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology. 2011; 140:1536–1546.
Article13. Cinghu S, Goh YM, Oh BC, et al. Phosphorylation of the gastric tumor suppressor RUNX3 following H. pylori infection results in its localization to the cytoplasm. J Cell Physiol. 2012; 227:1071–1080.14. Hamilton S, Aaltonen L. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press;2000.15. Lee SH, Kim TO, Lee DH, et al. Helicobacter pylori cagA, vacA, iceA Gene and Interleukin-1beta and Interleukin-1 receptor antagonist gene polymorphisms in gastric carcinoma. Korean J Med. 2006; 71:24–37.16. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999; 37:2274–2279.17. Chuang LS, Ito Y. RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene. 2010; 29:2605–2615.
Article18. Woolf E, Xiao C, Fainaru O, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003; 100:7731–7736.
Article19. Kim TY, Lee HJ, Hwang KS, et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004; 84:479–484.
Article20. Song HJ, Shim KN, Joo YH, Kim SE, Jung SA, Yoo K. Methylation of the tumor suppressor gene RUNX3 in human gastric carcinoma. Gut Liver. 2008; 2:119–125.
Article21. Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995; 55:2111–2115.22. Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998; 51:225–228.
Article23. Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010; 5:1885–1893.24. Sahara S, Sugimoto M, Vilaichone RK, et al. Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: a metaanalysis. BMC Infect Dis. 2012; 12:223.
Article25. Imamura Y, Hibi K, Koike M, et al. RUNX3 promoter region is specifically methylated in poorly-differentiated colorectal cancer. Anticancer Res. 2005; 25:2627–2630.26. Hibi K, Nakao A. Highly-methylated colorectal cancers show poorly-differentiated phenotype. Anticancer Res. 2006; 26:4263–4266.27. Li CQ, Pignatelli B, Ohshima H. Increased oxidative and nitrative stress in human stomach associated with cagA+ Helicobacter pylori infection and inflammation. Dig Dis Sci. 2001; 46:836–844.28. Wang YF, Guo CL, Zhao LZ, Yang GA, Chen P, Wang HK. Effect of Helicobacter pylori infection on gastric mucosal pathologic change and level of nitric oxide and nitric oxide synthase. World J Gastroenterol. 2005; 11:5029–5031.29. Katayama Y, Takahashi M, Kuwayama H. Helicobacter pylori causes runx3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochem Biophys Res Commun. 2009; 388:496–500.30. Lee SH, Kim J, Kim WH, Lee YM. Hypoxic silencing of tumor suppressor RUNX3 by histone modification in gastric cancer cells. Oncogene. 2009; 28:184–194.
Article31. Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011; 278:1190–1202.32. Liu Z, Xu X, Chen L, et al. Helicobacter pylori CagA inhibits the expression of Runx3 via Src/MEK/ERK and p38 MAPK pathways in gastric epithelial cell. J Cell Biochem. 2012; 113:1080–1086.33. Tsang YH, Lamb A, Chen LF. New insights into the inactivation of gastric tumor suppressor RUNX3: the role of H. pylori infection. J Cell Biochem. 2011; 112:381–386.34. Tammer I, Brandt S, Hartig R, König W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007; 132:1309–1319.35. Tsang YH, Lamb A, Romero-Gallo J, et al. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for protea-some-mediated degradation. Oncogene. 2010; 29:5643–5650.36. Subramaniam MM, Chan JY, Soong R, et al. RUNX3 inactivation in colorectal polyps arising through different pathways of colonic carcinogenesis. Am J Gastroenterol. 2009; 104:426–436.
Article37. Subramaniam MM, Chan JY, Soong R, et al. RUNX3 inactivation by frequent promoter hypermethylation and protein mislocalization constitute an early event in breast cancer progression. Breast Cancer Res Treat. 2009; 113:113–121.
Article38. Hsu PI, Hsieh HL, Lee J, et al. Loss of RUNX3 expression correlates with differentiation, nodal metastasis, and poor prognosis of gastric cancer. Ann Surg Oncol. 2009; 16:1686–1694.
Article39. Wei D, Gong W, Oh SC, et al. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005; 65:4809–4816.
Article40. Oshimo Y, Oue N, Mitani Y, et al. Frequent loss of RUNX3 expression by promoter hypermethylation in gastric carcinoma. Pathobiology. 2004; 71:137–143.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RUNX3 Methylation Status in Colonic Carcinoma and Adenoma
- An Inverse Relationship between the Expression of the Gastric Tumor Suppressor RUNX3 and Infection with Helicobacter pylori in Gastric Epithelial Dysplasia
- Methylation of the Tumor Suppressor Gene RUNX3 in Human Gastric Carcinoma
- The Role of Helicobacter pylori In fection and cagA Expression in Gastric Carcinogenesis
- DNA Methylation of RUNX3 in Papillary Thyroid Cancer