Korean J Gastroenterol.
2015 Jun;65(6):366-369. 10.4166/kjg.2015.65.6.366.
A Case of Disseminated Intra-abdominal Gastrointestinal Stromal Tumor Managed with Low Dose Imatinib
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea. gastro@catholic.ac.kr
- KMID: 2373239
- DOI: http://doi.org/10.4166/kjg.2015.65.6.366
Abstract
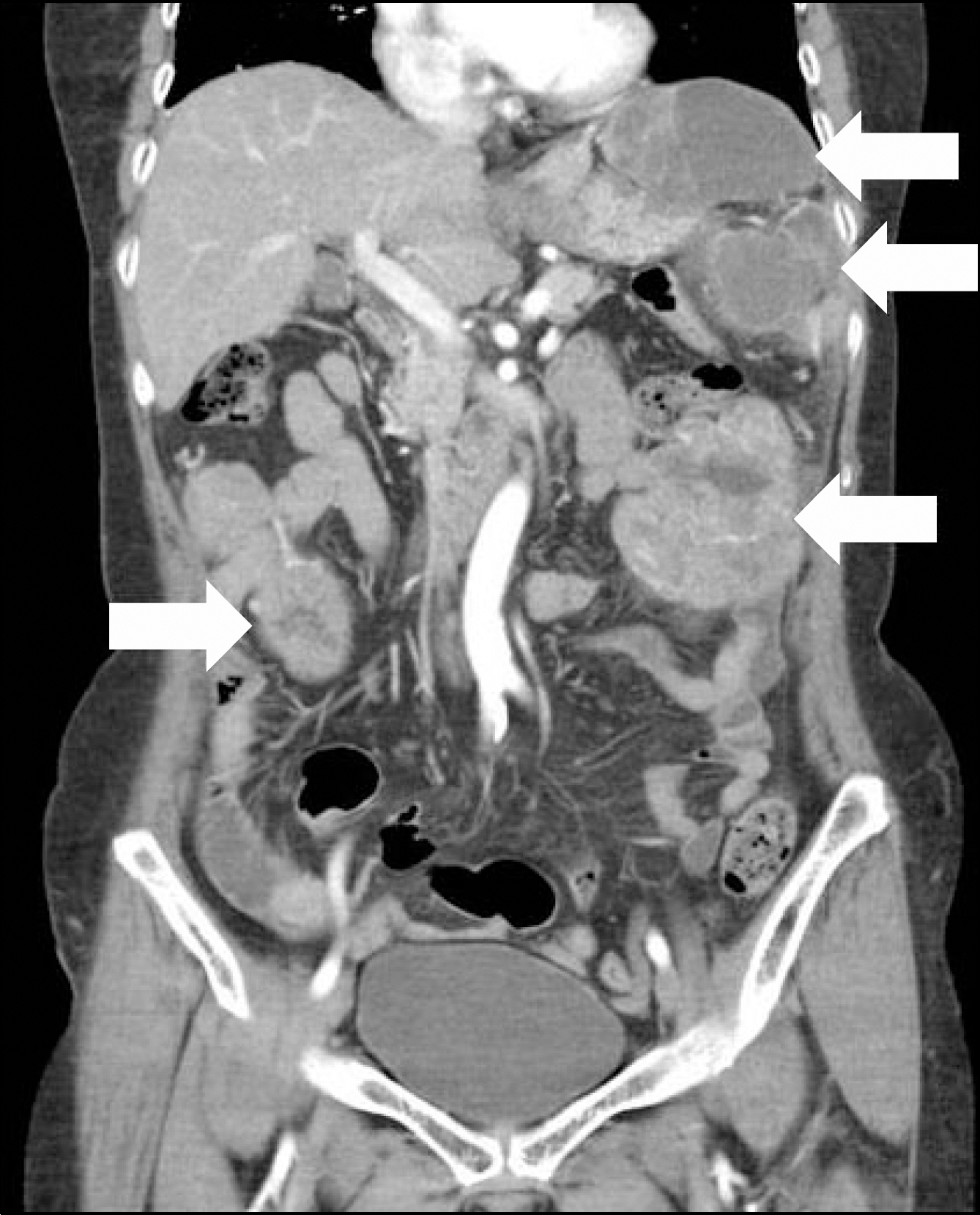
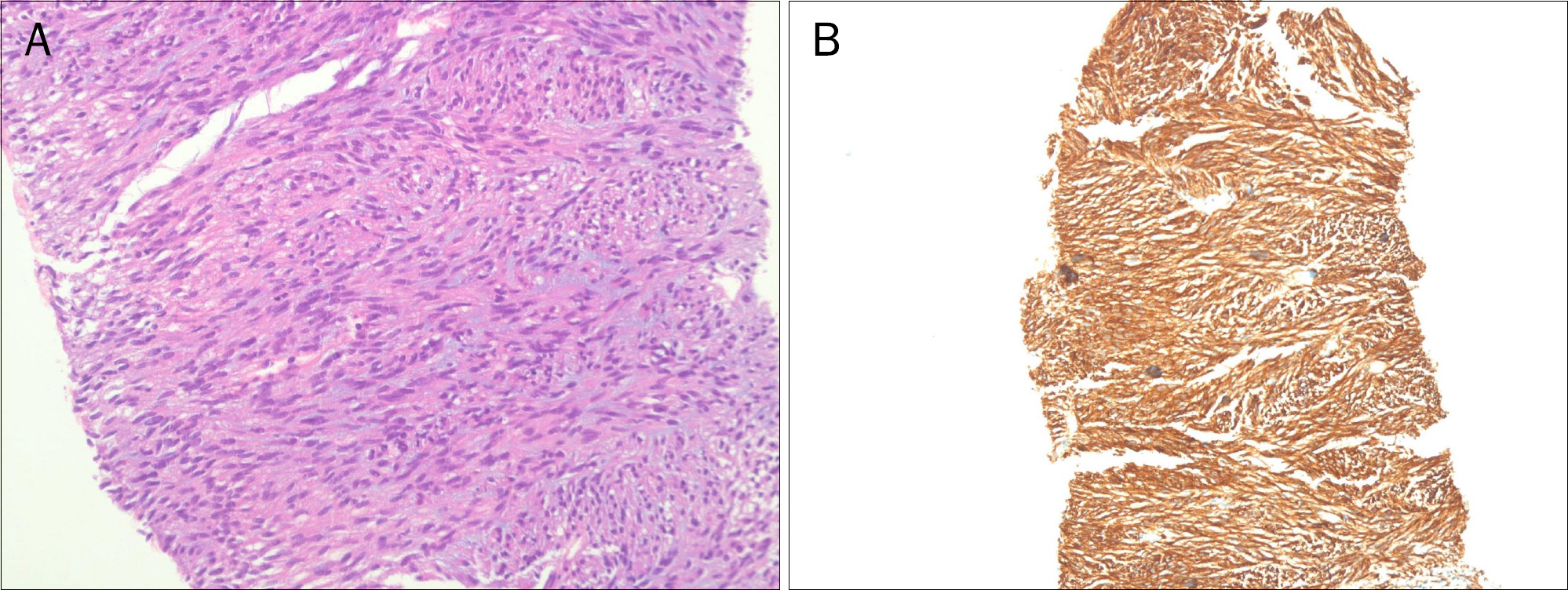
- Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract. Imatinib mesylate is recommended as adjuvant therapy for GIST after surgical resection. However, drug-related adverse events are common. A 74-year-old female with metastatic GIST who was managed with imatinib experienced severe adverse events, including skin rashes, tremor, and alopecia, etc. The imatinib dose was reduced and the size of the metastatic GIST continued to decrease and adverse events showed significant improvement.
Keyword
MeSH Terms
-
Aged
Antineoplastic Agents/adverse effects/*therapeutic use
Exanthema/etiology
Female
Gastrointestinal Neoplasms/diagnosis/*drug therapy/pathology
Gastrointestinal Stromal Tumors/diagnosis/*drug therapy/pathology
Humans
Imatinib Mesylate/adverse effects/*therapeutic use
Immunohistochemistry
Proto-Oncogene Proteins c-kit/metabolism
Tomography, X-Ray Computed
Antineoplastic Agents
Imatinib Mesylate
Proto-Oncogene Proteins c-kit
Figure
Reference
-
References
1. Reichardt P, Blay JY, Boukovinas I, et al. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol. 2012; 23:2776–2781.
Article2. Brouard M, Saurat JH. Cutaneous reactions to STI571. N Engl J Med. 2001; 345:618–619.
Article3. Valeyrie L, Bastuji-Garin S, Revuz J, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003; 48:201–206.
Article4. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.
Article5. Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010; 97:1854–1859.
Article6. Constantin VD, Socea B, Popa F, et al. A histopathological and immunohistochemical approach of surgical emergencies of GIST. An interdisciplinary study. Rom J Morphol Embryol. 2014; 55(2 Suppl):619–627.7. Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014; 12:269–280.
Article8. Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol. 2012; 9:351–358.
Article9. Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–1272.10. Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011; 37:75–88.
Article11. Hwang JE, Yoon JY, Bae WK, Shim HJ, Cho SH, Chung IJ. Imatinib induced severe skin reactions and neutropenia in a patient with gastrointestinal stromal tumor. BMC Cancer. 2010; 10:438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imatinib-induced DRESS Syndrome in Gastrointestinal Stromal Tumor
- Imatinib Plasma Monitoring-Guided Dose Modification for Managing Imatinib-Related Toxicities in Gastrointestinal Stromal Tumor Patients
- Imatinib-induced hepatitis treated by corticosteroids in a patient with metastatic gastrointestinal stromal tumor
- Successful Endoscopic Resection of a Rectal Gastrointestinal Stromal Tumor Larger Than 5 cm
- Two different KIT mutations may lead to different responses to imatinib in metastatic gastrointestinal stromal tumor