Korean J Gastroenterol.
2015 Apr;65(4):252-257. 10.4166/kjg.2015.65.4.252.
Pyogenic Pancreatic Abscess Mimicking Pancreatic Neoplasm: A Four-Case Series
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. smpark@chungbuk.ac.kr
- 2Department of Internal Medicine, Hana General Hospital, Cheongju, Korea.
- KMID: 2373218
- DOI: http://doi.org/10.4166/kjg.2015.65.4.252
Abstract
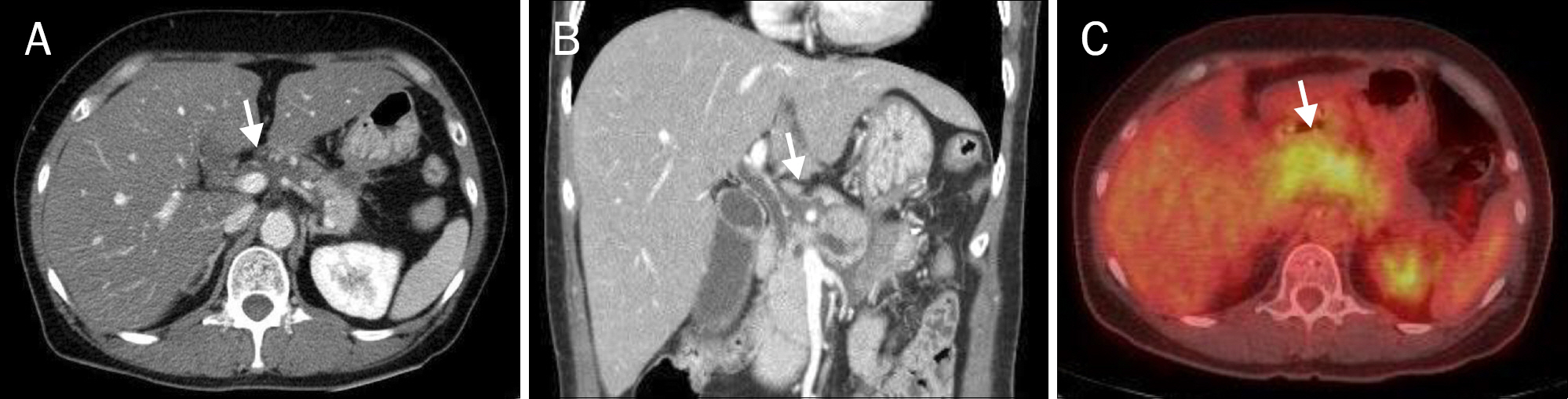
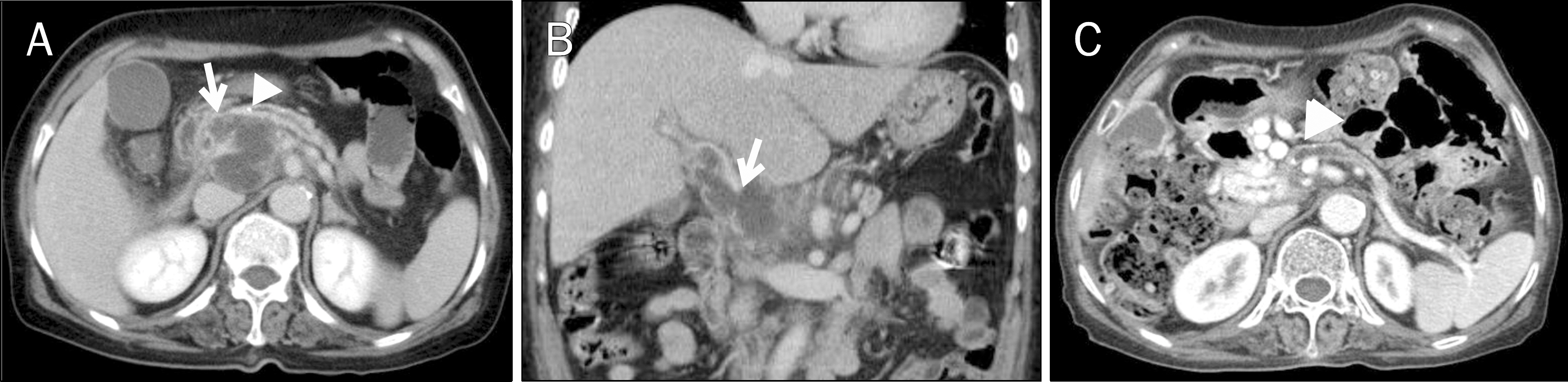
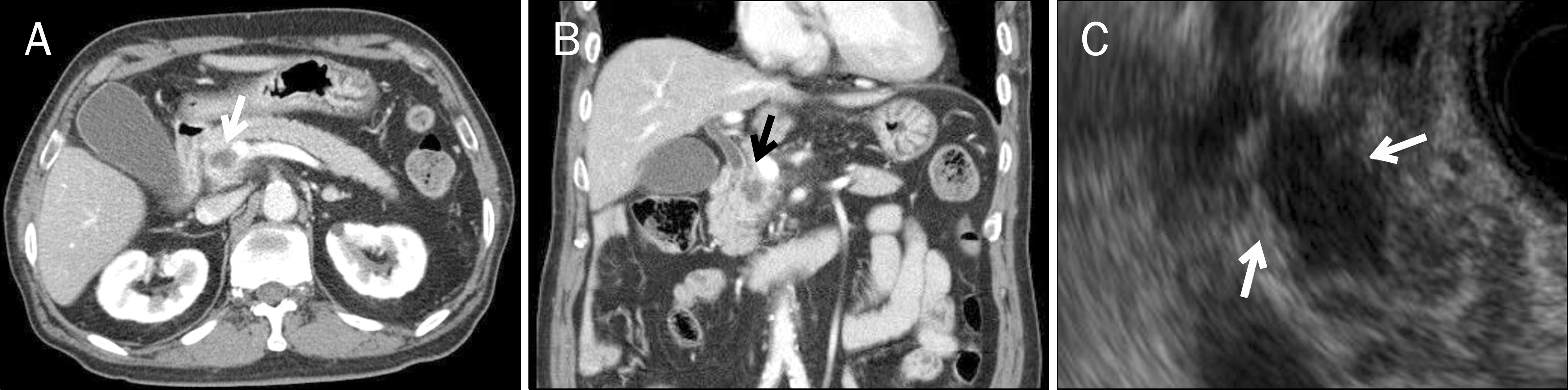
- A pyogenic pancreatic abscess mimicking pancreatic neoplasm in the absence of acute pancreatitis is rare. We report four patients who each presented with a pancreatic mass at the pancreas head or body without acute pancreatitis. The presenting symptoms were abdominal pain, fever, or weight loss. Abdominal CT scans showed low-density round masses at the pancreas head or body with/without lymphadenopathy. In each case, a PET-CT scan showed a mass with a high SUV, indicating possible malignancy. Comorbid diseases were identified in all patients: chronic pancreatitis and thrombus at the portal vein, penetrating duodenal ulcer, distal common bile duct stenosis, and diabetes mellitus. Diagnoses were performed by laparoscopic biopsy in two patients and via EUS fine needle aspiration in one patient. One patient revealed a multifocal microabscess at the pancreatic head caused by a deep-penetrating duodenal ulcer. He was treated with antibiotics and a proton-pump inhibitor. The clinical symptoms and pancreatic images of all the patients were improved using conservative management. Infective causes should be considered for a pancreatic mass mimicking malignancy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Goh BK, Jeyaraj PR, Chan HS, et al. A case of fish bone perforation of the stomach mimicking a locally advanced pancreatic carcinoma. Dig Dis Sci. 2004; 49:1935–1937.2. An CH, Kim KH, Kim JS, Kim JI. Pancreatic abscess caused by gastric perforation. ANZ J Surg. 2007; 77:709.
Article3. Saxon A, Reynolds JT, Doolas A. Management of pancreatic abscesses. Ann Surg. 1981; 194:545–552.
Article4. Chong VH. Isolated pyogenic pancreatic abscess mimicking a neoplasm. JOP. 2008; 9:309–312.5. Safioleas M, Stamatakos MK, Mouzopoulos GJ, et al. Pancreatic abscess due to perforation of duodenal diverticulum. Chirurgia (Bucur). 2006; 101:523–524.6. Hu J, Sheu MH, Yang WC, Li JC, Ng YY. Peritonitis and pancreatic abscess in a CAPD patient. Perit Dial Int. 2002; 22:430–431.
Article7. Seo KS, Oh HM, Hong JH, et al. A case of pancreatic abscess due to Salmonella typhi. Korean J Med. 1998; 54:101–104.8. Arya M, Arya PK. Pancreatic abscess caused by s. typhi. Indian J Med Microbiol. 2001; 19:18–19.
Article9. Garg P, Parashar S. Pancreatic abscess due to Salmonella typhi. Postgrad Med J. 1992; 68:294–295.
Article10. Taguchi M, Nishikawa S, Matsuoka H, et al. Pancreatic abscess caused by Corynebacterium coyleae mimicking malignant neoplasm. Pancreas. 2006; 33:425–429.
Article11. Eliashiv A, Olumide F, Norton L, Eiseman B. Depression of cell-mediated immunity in diabetes. Arch Surg. 1978; 113:1180–1183.
Article12. Chase MP, Yarze JC, Gumustop B, Leach RP. Endoscopic ultrasound-guided aspiration and oral antibiotic therapy as definitive treatment of an asymptomatic pancreatic abscess. Pancreas. 2009; 38:475–476.
Article13. Horvath KD, Kao LS, Wherry KL, Pellegrini CA, Sinanan MN. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001; 15:1221–1225.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Pancreatic Tuberculosis Masquerading as a Pancreatic Mass
- Isolated Pyogenic Pancreatic Abscess Successfully Treated via Endoscopic Ultrasound-guided Drainage
- Percutaneous drainage of pancreatic abscess and pseudocyst
- A Case of Pancreatic Abscess Due to Salmonella Typhi
- Acute Ischemic Hepatitis and Pancreatic Abscess after Elective Abdominal Aortic Aneurysm Repair: A Case Report