J Pathol Transl Med.
2017 Mar;51(2):159-164. 10.4132/jptm.2016.07.01.
A Pyloric Gland-Phenotype Ovarian Mucinous Tumor Resembling Lobular Endocervical Glandular Hyperplasia in a Patient with Peutz-Jeghers Syndrome
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. krkim@amc.seoul.kr
- 2Department of Medical Genetics, Asan Medical Center Children’s Hospital, Seoul, Korea.
- KMID: 2372965
- DOI: http://doi.org/10.4132/jptm.2016.07.01
Abstract
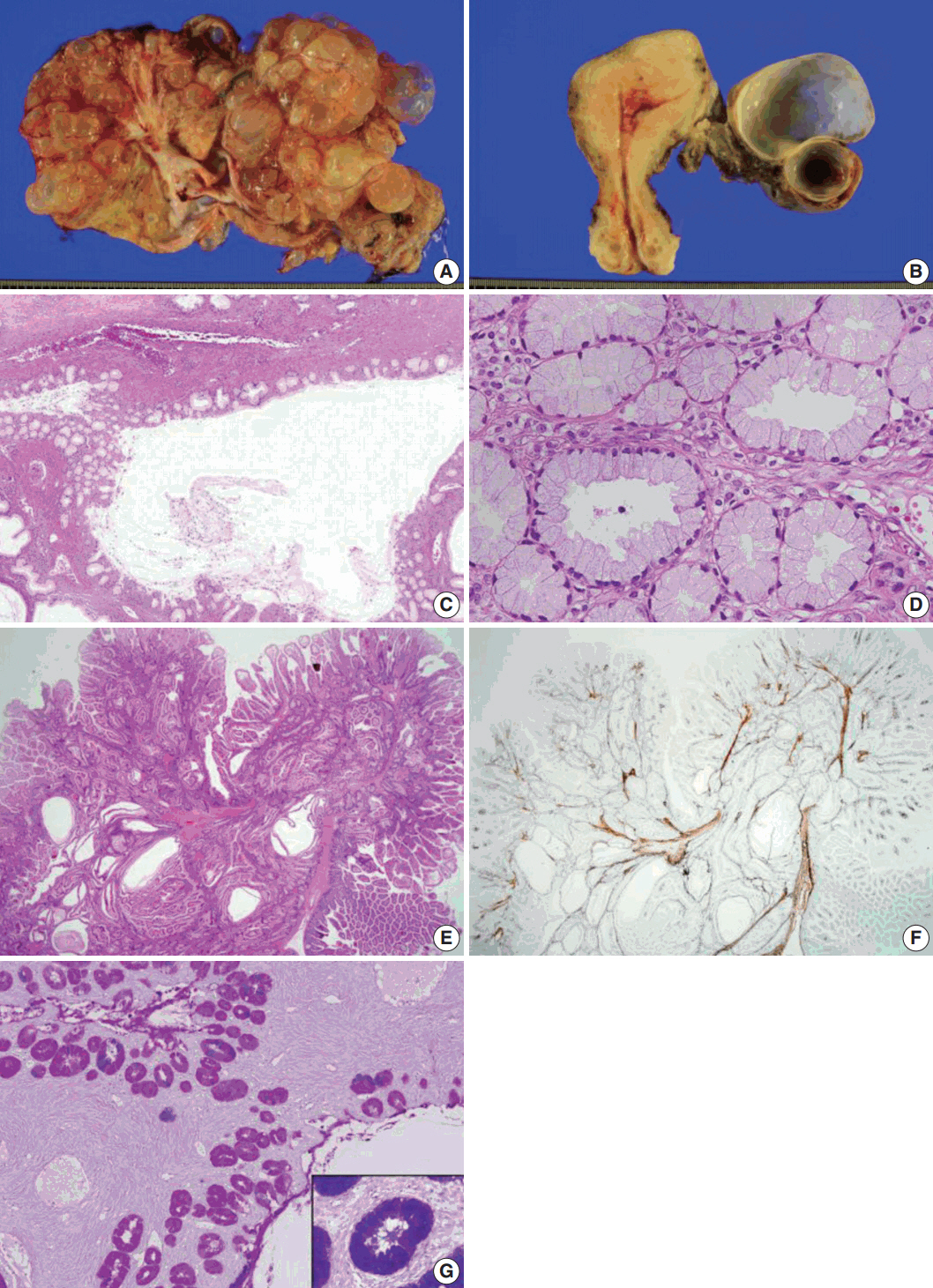
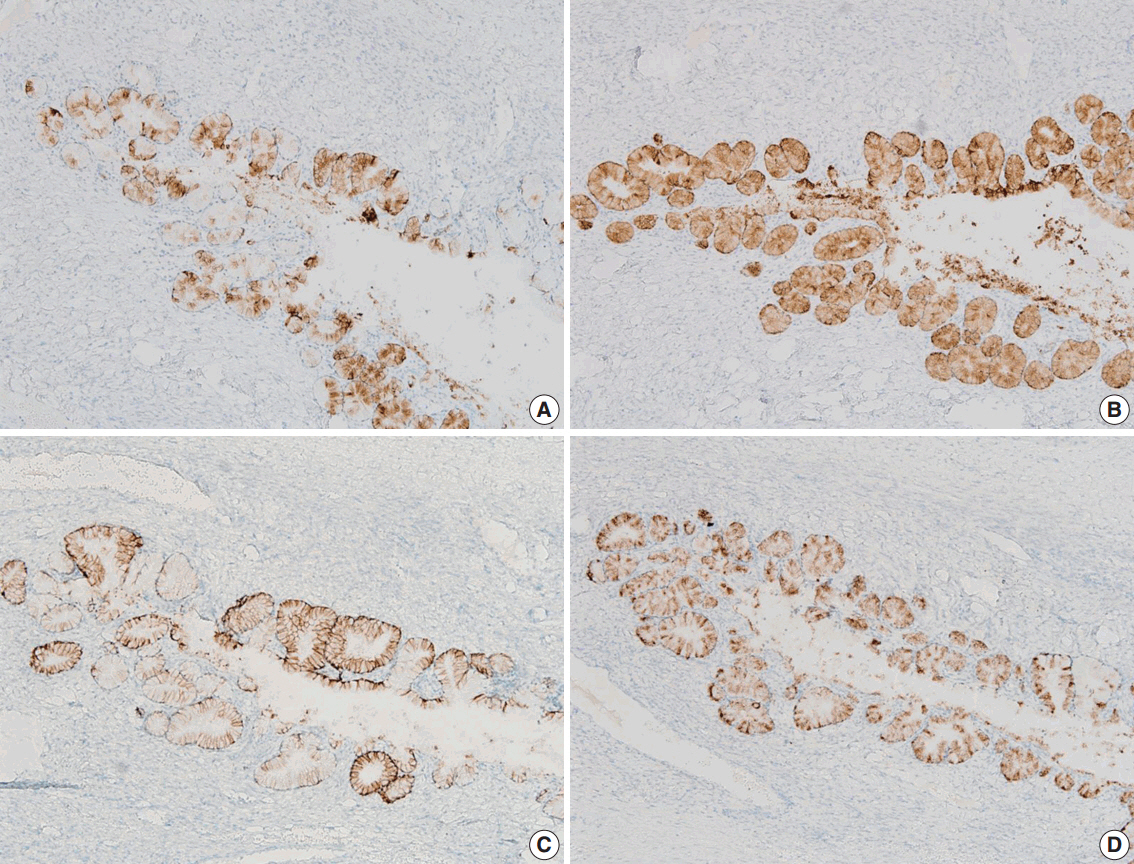
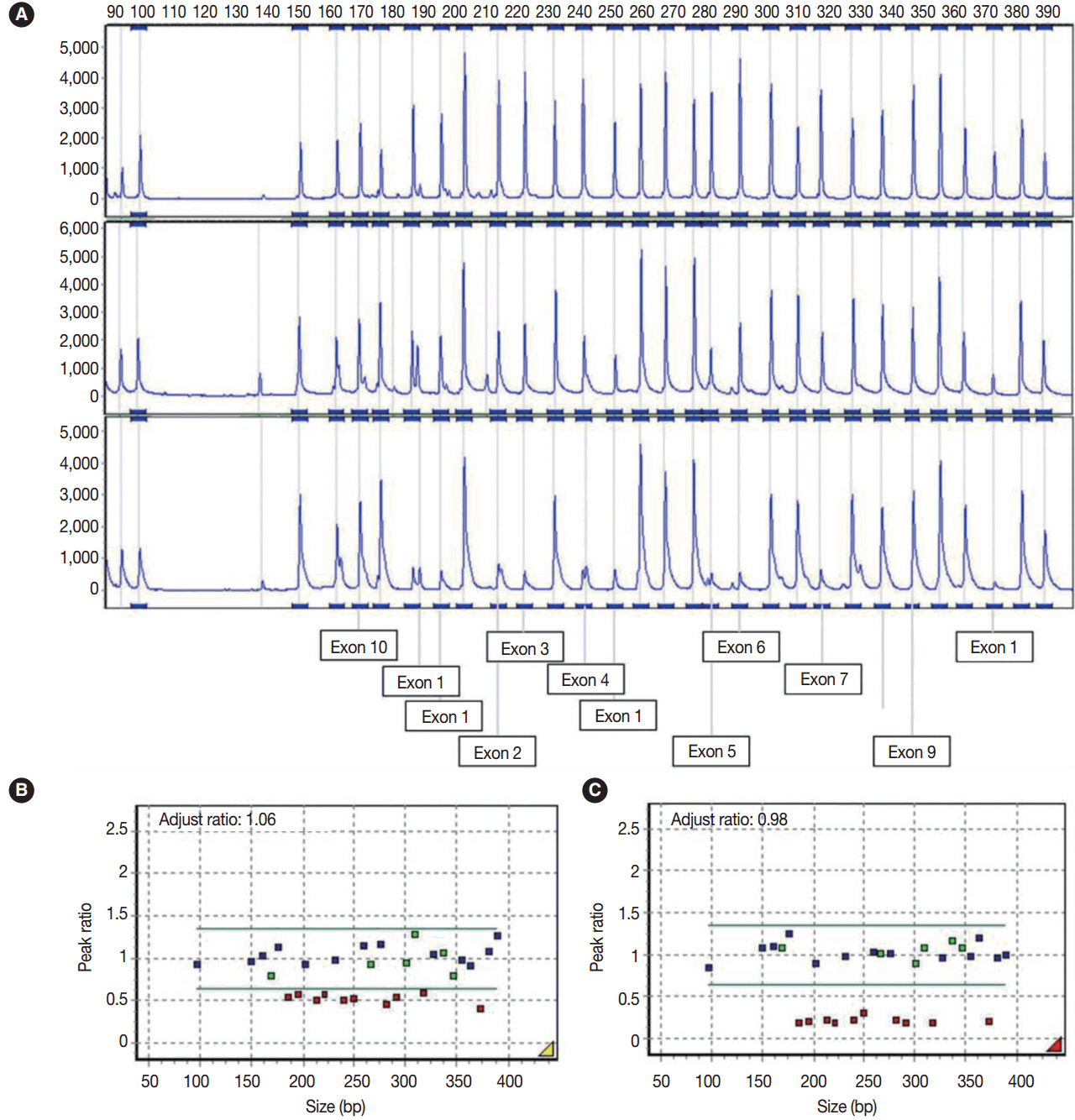
- We describe an ovarian mucinous neoplasm that histologically resembles lobular endocervical glandular hyperplasia (LEGH) containing pyloric gland type mucin in a patient with Peutz-Jeghers syndrome (PJS). Although ovarian mucinous tumors rarely occur in PJS patients, their pyloric gland phenotype has not been clearly determined. The histopathologic features of the ovarian mucinous tumor were reminiscent of LEGH. The cytoplasmic mucin was stained with periodic acid-Schiff reaction after diastase treatment but was negative for Alcian blue pH 2.5, suggesting the presence of neutral mucin. Immunohistochemically, the epithelium expressed various gastric markers, including MUC6, HIK1083, and carbonic anhydrase-IX. Multiple ligation-dependent probe amplification detected a germline heterozygous deletion mutation at exons 1-7 of the STK11 gene (c.1-?_920+?del) in peripheral blood leukocytes and mosaic loss of heterozygosity in ovarian tumor tissue. Considering that LEGH and/or gastric-type cervical adenocarcinoma can be found in patients with PJS carrying germline and/or somatic STK11 mutations, our case indicates that STK11 mutations have an important role in the proliferation of pyloric-phenotype mucinous epithelium at various anatomical locations.
Keyword
Figure
Reference
-
1. Ferry JA, Young RH, Engel G, Scully RE. Oxyphilic Sertoli cell tumor of the ovary: a report of three cases, two in patients with the Peutz-Jeghers syndrome. Int J Gynecol Pathol. 1994; 13:259–66.
Article2. Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998; 18:38–43.3. Kuragaki C, Enomoto T, Ueno Y, et al. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest. 2003; 83:35–45.
Article4. Kato N, Sugawara M, Maeda K, Hosoya N, Motoyama T. Pyloric gland metaplasia/differentiation in multiple organ systems in a patient with Peutz-Jegher’s syndrome. Pathol Int. 2011; 61:369–72.
Article5. Kondo T, Hashi A, Murata S, et al. Endocervical adenocarcinomas associated with lobular endocervical glandular hyperplasia: a report of four cases with histochemical and immunohistochemical analyses. Mod Pathol. 2005; 18:1199–210.
Article